2141‑V11 Anti‑CD40 Antibody Shows Promise in Metastatic Cancer

The 2141-V11 anti-CD40 antibody in metastatic cancer has shown strong safety and promising results in early trials. Most patients experienced only mild side effects, such as fever or chills, and no serious toxicities appeared. In a group of twelve patients, two achieved complete responses lasting a median of twelve months. This therapy stands out because it can trigger the growth of new immune structures, called tertiary lymphoid structures, inside tumors. These structures help the body attack cancer cells both in treated and untreated areas.
Key Takeaways
The 2141-V11 anti-CD40 antibody is safe and well tolerated, with mostly mild side effects like fever and injection site reactions.
This therapy can shrink tumors and even cause complete cancer disappearance in some patients, with effects lasting many months.
2141-V11 works by activating the immune system inside tumors, creating special immune hubs called tertiary lymphoid structures that boost cancer-fighting cells.
The antibody triggers immune responses not only in treated tumors but also in distant tumors, showing strong systemic effects.
Ongoing research explores combining 2141-V11 with other treatments to improve patient outcomes and develop new cancer immunotherapy strategies.
2141-V11 anti-CD40 antibody in metastatic cancer: Safety

Adverse Events
The safety profile of the 2141-V11 anti-CD40 antibody in metastatic cancer has been well characterized in early clinical trials. Researchers treated twelve patients with intratumoral injections every three weeks. Most patients tolerated the therapy well. The majority of adverse events were mild or moderate. No dose-limiting toxicities appeared during the study.
The following table summarizes the frequency and severity of adverse events observed in the trial:
Parameter | Data |
|---|---|
Number of participants | |
Treatment | Intratumoral 2141-V11 every 3 weeks |
Patients with any adverse events | 10/12 (83%) |
Patients with treatment-related adverse events (TRAEs) | 7/12 (58%) |
Highest grade of TRAEs | Grade 3 (no grade 4 or 5 reported) |
Number of severe adverse events | 3 (none treatment-related) |
Common mild side effects | Fever, injection site reactions |
Dose-limiting toxicities | None observed |
Liver and platelet levels | Stable during treatment |
Safety profile | Well tolerated, manageable toxicity |
Patients most often reported fever and reactions at the injection site. Three severe adverse events occurred, but none were related to the treatment. Liver and platelet levels stayed stable throughout therapy. The absence of dose-limiting toxicities supports the manageable safety profile of the 2141-V11 anti-CD40 antibody in metastatic cancer.
In a separate study involving patients with non-muscle invasive bladder cancer, similar results appeared. Over half of the patients experienced treatment-related adverse events, but only one grade 3 event was reported. No dose-limiting toxicities occurred, and the therapy remained well tolerated.
Researchers also observed some immune-related adverse events. The most common included hepatotoxicity, cytokine release syndrome, and thrombocytopenia. These effects are typical for CD40 agonists. The antibody’s design helps reduce these risks by using intratumoral delivery instead of systemic dosing.
Immunogenicity
The development of anti-drug antibodies can affect the safety and effectiveness of antibody therapies. In trials with the 2141-V11 anti-CD40 antibody in metastatic cancer, scientists monitored patients for signs of immunogenicity. Some patients developed anti-drug antibodies, but these did not cause serious immune reactions or reduce the therapy’s effectiveness.
Clinical investigators noted that the antibody’s Fc-engineered structure did not increase the risk of severe immune responses. No cases of anaphylaxis or severe hypersensitivity appeared. The absence of serious immunogenicity supports the continued development of this therapy.
Preclinical studies in animal models predicted possible toxicities at higher systemic doses, such as liver toxicity and thrombocytopenia. By using intratumoral injections and careful dose escalation, researchers avoided these problems in human trials. The safety data from both clinical and preclinical studies confirm that the 2141-V11 anti-CD40 antibody in metastatic cancer offers a favorable safety profile.
Antitumor Activity
Tumor Response
Clinical trials have shown that the 2141-V11 anti-CD40 antibody in metastatic cancer can shrink tumors in a significant number of patients. In a recent phase 1 study, researchers treated twelve patients and evaluated eleven for tumor response. Six patients saw their tumors shrink, and two patients achieved a complete response, meaning their cancer disappeared entirely. These complete responses occurred in patients with melanoma and breast cancer, both known for aggressive behavior.
The median duration of response for patients who experienced tumor regression reached 12 months, with some responses lasting over 16 months. This suggests that the benefits of treatment can be long-lasting for those who respond.
The following table summarizes the tumor responses observed in the trial:
Measure | Number of Patients/Lesions | Response Type |
|---|---|---|
Total patients treated | 12 | N/A |
Patients evaluable for response | 11 | N/A |
Patients with tumor burden decrease | 6 | Partial or better |
Patients with disease control | 4 | Partial or better |
Patients with complete response | 2 | Complete response |
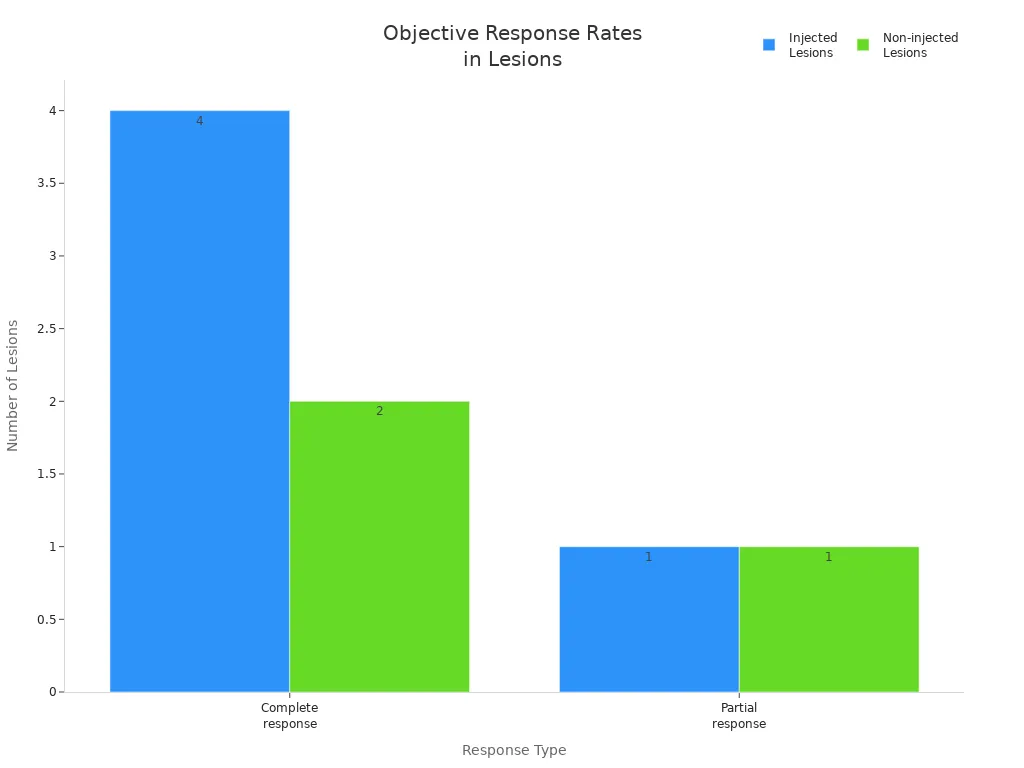
Injected lesions evaluated | 16 | N/A |
Injected lesions with complete response | 4 | Complete response |
Injected lesions with partial response | 1 | Partial response |
Non-injected lesions evaluated | 12 | N/A |
Non-injected lesions with complete response | 2 | Complete response |
Non-injected lesions with partial response | 1 | Partial response |
Systemic Effects
The antitumor effects of this therapy extend beyond the injected tumors. In the phase 1 trial, doctors observed that tumors far from the injection site also shrank or disappeared. This systemic response points to strong immune activation throughout the body.
Six patients experienced tumor shrinkage, not just in the injected area but also in distant tumors.
Two patients, one with melanoma and one with breast cancer, achieved complete responses. Their cancers vanished from all sites, including skin, liver, and lung.
In one case, a patient with multiple melanoma tumors received an injection in only one tumor. All other tumors regressed, showing a powerful systemic effect.
Another patient with breast cancer had tumors in several organs. After a single injection into a skin tumor, all tumors disappeared.
Researchers found new immune structures, called tertiary lymphoid structures (TLS), in both injected and non-injected tumors. These structures help the immune system attack cancer cells more effectively.
The therapy was designed for direct injection into tumors. This approach reduced side effects and improved the immune response.
These results suggest that the 2141-V11 anti-CD40 antibody in metastatic cancer can trigger the body’s immune system to fight cancer throughout the body, not just at the injection site. The formation of TLS in distant tumors supports this systemic effect.
While these findings are promising, researchers note that more studies are needed to confirm the long-term safety and effectiveness of this approach. Earlier CD40 antibodies caused serious side effects, but the new method of direct tumor injection has improved safety. The broader use of this therapy and its long-term impact remain under investigation.
Mechanism and Immune Response
Immune Activation
The 2141-V11 anti-CD40 antibody in metastatic cancer works by activating the body’s immune system in a unique way. Its Fc-engineered design allows it to bind strongly to FcγRIIB receptors. This binding helps the antibody crosslink and activate CD40 receptors on antigen-presenting cells, such as dendritic cells, B cells, and macrophages. When doctors inject the antibody directly into a tumor, these cells become more active. They present tumor antigens to T cells, which then recognize and attack cancer cells. This process increases the number of CD8+ T cells and B cells inside the tumor. The antibody’s local delivery also helps limit side effects while still creating a strong immune response throughout the body.
Tertiary Lymphoid Structures
Tertiary lymphoid structures, or TLS, are special immune hubs that form inside tumors after treatment. Researchers have seen that the 2141-V11 anti-CD40 antibody in metastatic cancer can cause these structures to appear in both animal models and patients.
In mouse models, the antibody led to complete tumor regression and the formation of TLS.
TLS bring together B cells and dendritic cells, creating a space for immune cells to interact.
These structures help recruit more immune cells to the tumor, making the immune attack stronger.
TLS formation has been linked to tumor shrinkage in both injected and non-injected tumors.
Clinical studies show that patients with mature TLS often have better responses to treatment.
Key Immune Cells
TLS contain many types of immune cells that work together to fight cancer.
Cell Type | Role in TLS and Tumor Immunity |
|---|---|
CD8+ T cells | Attack and kill cancer cells directly |
B cells | Produce antibodies and help present antigens |
Dendritic cells | Activate T cells by showing them tumor antigens |
mregDCs (mature regulatory dendritic cells) | Support T cell activation and help shape the immune response |
These cells create a powerful team. CD8+ T cells and B cells increase in number after treatment, leading to better tumor control. The presence of TLS and these key cells often means a stronger and longer-lasting response to therapy.
Preclinical Insights
Mouse Model Results
Researchers tested the 2141-V11 anti-CD40 antibody in CD40/Fcγ receptor humanized mice with tumors. Intratumoral injections of 2141-V11 led to strong tumor regression. The antibody promoted the formation of tertiary lymphoid structures (TLS) inside the tumors. These structures helped boost the number and activity of CD8+ T cells, which attack cancer cells directly. Mice treated with 2141-V11 showed not only shrinkage of the injected tumors but also control of tumors at distant sites. This effect, called the abscopal effect, means the immune system fought cancer throughout the body.
Intratumoral 2141-V11 at the maximum tolerated dose controlled both treated and untreated tumors.
The systemic antitumor activity depended on Fc receptor binding and CD8+ T cells.
Lower doses of 2141-V11 reduced tumor burden more than systemic delivery, with no liver or platelet toxicity.
Mice cured by 2141-V11 resisted tumor rechallenge, showing long-lasting immunity.
Depletion studies confirmed that CD8+ T cells were essential for tumor control, while macrophages and NK cells were not.
An Fc-mutant version of 2141-V11 that could not bind Fc receptors failed to stop tumor growth.
These results show that 2141-V11 can create a strong and lasting immune response in mouse models.
Immune Cell Infiltration
The preclinical studies revealed that 2141-V11 increased the presence of key immune cells inside tumors. After treatment, researchers found more CD8+ and CD4+ T cells, as well as B cells, within the tumor microenvironment. The formation of TLS provided a space for these cells to interact and coordinate an attack on cancer cells. Mice that responded to treatment showed expansion of effector and memory CD8+ T cells, which are important for long-term protection.
Clinical trials in humans mirrored these findings. Patients who responded to 2141-V11 had increased immune cell infiltration and TLS formation in their tumors. The antibody’s Fc-optimized design improved its ability to activate the immune system while reducing side effects. Both preclinical and clinical data support the idea that 2141-V11 can drive a powerful and lasting immune response against cancer.
Future Directions
Clinical Development
Clinical trials continue to expand the understanding of CD40 agonist therapies. Investigators have launched a phase 1 study to evaluate intratumoral administration of the Fc-optimized agonistic anti-CD40 antibody 2141-V11. The trial enrolls patients with locally advanced or metastatic solid tumors. Researchers use dose escalation levels of 0.7 mg, 2.0 mg, 7.0 mg, and 10 mg every three weeks. The primary endpoints focus on safety, maximum tolerated dose, and recommended phase 2 dose. Secondary endpoints include preliminary clinical activity and correlative studies from biospecimens. The trial, registered as NCT04059588, reports that the therapy is well tolerated. No dose-limiting toxicities have appeared, and the maximum tolerated dose has not been reached. The overall response rate stands at 20%, including complete responses and stable disease.
Aspect | Details |
|---|---|
Trial Phase | Phase 1 |
Intervention | Intratumoral administration of Fc-optimized agonistic anti-CD40 antibody 2141-V11 |
Patient Population | Patients with locally advanced or metastatic solid tumors |
Dose Escalation Levels | 0.7 mg, 2.0 mg, 7.0 mg, 10 mg every 3 weeks |
Primary Endpoints | Safety, Maximum Tolerated Dose (MTD), Recommended Phase 2 Dose |
Secondary Endpoints | Preliminary clinical activity, correlative studies from biospecimens |
Safety Results | Well tolerated, no dose-limiting toxicities, MTD not reached |
Clinical Activity | Overall response rate 20% (including complete responses and stable disease) |
Trial Registration Number | NCT04059588 |
Research Outlook
Researchers explore combination strategies to improve outcomes for patients. They combine CD40 agonists with IL-15 to boost antitumor immunity and memory T cell responses. Chemotherapy increases neoantigen availability and enhances immune activation. Immune checkpoint inhibitors, such as PD-1/PD-L1 and CTLA-4 inhibitors, further stimulate T-cell activity. Antiangiogenic drugs normalize tumor blood vessels and help T cells enter tumors, especially in immunologically cold tumors. Gene therapy approaches, including oncolytic viruses delivering CD40 agonists, also show promise. Clinical trials that combine CD40 agonists with chemotherapy and checkpoint inhibitors report encouraging response rates and long-term survival in difficult cancers.
Preclinical studies show that pairing 2141-V11 with IL-15 leads to durable systemic antitumor immunity. This combination enriches memory CD8 T cells and mature NK cells, resulting in long-term survival and rejection of tumor rechallenge. The effect resembles in situ vaccination.
The induction of tertiary lymphoid structures (TLS) stands out as a novel and promising strategy. Mature TLS contain germinal centers with activated B cells, which produce high-affinity antibodies. These structures predict better prognosis and stronger anti-tumor immune responses. Therapies that promote TLS formation, such as immunotherapy, chemoradiotherapy, and vaccines, convert cold tumors into hot tumors. This change increases cytotoxic T cell infiltration and improves treatment outcomes. Researchers focus on optimizing delivery, patient selection, and broadening indications to enhance safety and effectiveness.
The future of cancer immunotherapy may rely on strategies that combine enhancement of tumor antigenicity with stimulation of TLS formation. This approach could strengthen cytotoxic T cell responses and improve patient outcomes.
Clinical trials show that the 2141-V11 anti-CD40 antibody in metastatic cancer offers a strong safety profile and durable tumor responses. The antibody’s Fc-engineered design activates dendritic cells and CD8+ T cells, leading to the formation of tertiary lymphoid structures. These structures boost local and systemic immunity, resulting in complete responses and long-term tumor control. Researchers continue to explore combination therapies and new delivery methods to improve outcomes for patients.
The induction of TLS and systemic immunity marks a major advance in cancer immunotherapy.
FAQ
What is the 2141-V11 anti-CD40 antibody?
2141-V11 is a laboratory-made antibody. It targets the CD40 protein on immune cells. Doctors inject it directly into tumors. This approach helps the immune system attack cancer cells more effectively.
How safe is 2141-V11 for patients?
Clinical trials show that 2141-V11 is well tolerated. Most patients report mild side effects like fever or injection site reactions. No dose-limiting toxicities have appeared. Doctors monitor patients closely during treatment.
What makes 2141-V11 different from other cancer therapies?
2141-V11 induces tertiary lymphoid structures (TLS) inside tumors. These structures help the immune system fight cancer both locally and throughout the body. This effect sets 2141-V11 apart from many other immunotherapies.
Can 2141-V11 work with other treatments?
Researchers study 2141-V11 in combination with chemotherapy, immune checkpoint inhibitors, and IL-15. Early results suggest that these combinations may improve immune responses and help more patients.
Who might benefit most from 2141-V11 therapy?
Patients with metastatic or advanced solid tumors may benefit. Doctors select patients based on tumor type and immune features. Ongoing trials will help identify those who respond best.
See Also
Recognizing Symptoms And Treatment Options For Duodenal Cancer
Anaplastic Large Cell Lymphoma: Causes And Treatment Methods
A Guide To Large Cell Lung Cancer With Rhabdoid Features
Treatment Approaches For Acute Myeloid Dendritic Cell Leukemia

