How 3D Bioprinting is Shaping the Future of Personalized Medicine
Imagine a world where you no longer have to wait years for an organ transplant or rely on generic treatments that may not suit your unique biology. 3D bioprinting is making this vision a reality. By creating patient-specific tissues and organs, this technology is revolutionizing healthcare.
Here are some numbers that highlight its growing impact:
The global precision medicine market, driven by advancements like 3D bioprinting, generated $83.4 billion in 2022 and is projected to reach $254 billion by 2032.
In drug testing, 3D bioprinting was valued at $580 million in 2020, with expectations to grow to $1.9 billion by 2027.
The organ-on-chip market, which uses this technology, is growing at an impressive annual rate of 37.6%.
These advancements mark the beginning of a new horizon in healthcare—one where 3D Bioprinting for Personalized Medicine: A New Horizon is no longer a distant dream but a transformative reality.
Key Takeaways
3D bioprinting makes custom organs and tissues. It lowers transplant wait times and rejection chances.
This tech improves drug testing by using human-like tissues. It helps create new treatments faster.
Custom implants and prosthetics from bioprinting fit better. They are made to match each person’s body.
3D bioprinting helps wounds heal faster with special scaffolds and skin patches. It changes how wounds are treated.
The future of healthcare looks great with 3D bioprinting. It can solve organ shortages and make treatments more accurate.
3D Bioprinting for Personalized Medicine: A New Horizon

What is 3D Bioprinting?
3D bioprinting is an advanced technology that uses living cells and biomaterials to create three-dimensional structures. These structures mimic natural tissues and organs, offering solutions for medical challenges like organ shortages and ineffective treatments. By layering bioinks—materials made from living cells—this process builds functional tissues that can repair or replace damaged areas in the body.
You might wonder why this matters. Imagine a future where doctors can create a custom organ for you using your own cells. This approach eliminates the risk of organ rejection and reduces the need for long transplant waiting lists. Personalized medicine becomes more effective because treatments are tailored to your unique biology.
How Does 3D Bioprinting Work?
3D bioprinting involves several steps, starting with a digital blueprint of the tissue or organ. This blueprint guides the printer as it deposits layers of bioink to form the desired structure. The process requires precision and advanced techniques to ensure the final product functions like natural tissue.
Techniques in 3D Bioprinting
You’ll find that different techniques are used depending on the application. Inkjet bioprinting sprays tiny droplets of bioink onto a surface, creating intricate patterns. Extrusion bioprinting uses a nozzle to deposit continuous streams of bioink, ideal for building larger structures. Laser-assisted bioprinting employs focused light to position cells with extreme accuracy. Each method has its strengths, allowing researchers to tackle various medical challenges.
The Role of Bioinks
Bioinks play a critical role in 3D bioprinting. These materials combine living cells with supportive substances like hydrogels. The bioink must provide a suitable environment for cells to grow and function. For example, bioinks made from a patient’s cells can create personalized tissue constructs. This approach enhances treatment effectiveness and reduces adverse reactions during drug testing.
Key Components of the Technology
Several components make 3D bioprinting possible. The printer itself is a sophisticated machine capable of precise movements. Bioinks, as mentioned earlier, are essential for creating functional tissues. Digital models guide the printing process, ensuring accuracy. Together, these elements enable the creation of artificial biological structures that mimic natural tissues. This technology supports applications like organ transplantation, drug testing, and tissue repair, paving the way for a new era in personalized medicine.
Applications in Personalized Medicine
Organ and Tissue Printing
Functional Organs for Transplantation
Imagine a future where you no longer have to wait years for an organ transplant. 3D bioprinting makes this possible by creating functional organs tailored to your body. Using your own cells, scientists can bioprint organs like kidneys or livers that match your unique biology. This reduces the risk of rejection and eliminates the need for immunosuppressive drugs. For patients with organ failure, this technology offers a life-saving solution.
Custom Tissues for Research and Therapy
Custom tissues created through 3D bioprinting are transforming medical research. These tissues mimic human biology, allowing researchers to study diseases more accurately. For example, you can test how a specific treatment affects your cells before undergoing therapy. This approach not only improves treatment outcomes but also accelerates the development of new therapies.
Drug Development and Delivery
Bioprinted Models for Drug Testing
Traditional drug testing often relies on animal models, which may not accurately predict how a drug will work in humans. With 3D bioprinted models, you can test drugs on tissues that closely resemble human organs. This improves accuracy and reduces the time needed to bring new drugs to market. It also minimizes the ethical concerns associated with animal testing.
Personalized Drug Delivery Systems
3D bioprinting enables the creation of drug delivery systems tailored to your needs. These systems can release medication at a controlled rate, ensuring optimal effectiveness. Compared to conventional methods, bioprinted systems offer several advantages:
Advantage | 3D Bioprinted Systems | Conventional Methods |
|---|---|---|
Controlled Release Kinetics | Yes | No |
Personalized Medicine | Yes | No |
Cost-Effectiveness | Yes | Limited |
Integration of Multiple Drugs | Yes | No |
Tailored to Patient Needs | Yes | No |
This personalized approach ensures that you receive the right dose at the right time, improving treatment outcomes.
Regenerative Medicine
Bioprinted Scaffolds for Tissue Repair
When you suffer from tissue damage, bioprinted scaffolds can help your body heal. These scaffolds provide a structure for cells to grow and regenerate damaged tissues. For example, they can repair cartilage in your joints or restore damaged heart tissue. This approach enhances the body’s natural healing process and reduces recovery time.
Advancing Wound Healing and Skin Regeneration
Severe wounds and burns often require skin grafts, which can be painful and time-consuming. 3D bioprinting offers a less invasive alternative. By using your cells, scientists can create skin grafts that match your skin’s texture and color. These grafts promote faster healing and reduce the risk of complications. For patients with chronic wounds, this technology provides a revolutionary solution.
Patient-Specific Medical Devices
Custom Implants and Prosthetics
3D bioprinting is revolutionizing the creation of implants and prosthetics tailored to your unique anatomy. Traditional implants often require adjustments to fit properly, but bioprinted devices eliminate this issue. By using your medical scans, scientists can design implants that match your exact dimensions. This ensures better comfort, functionality, and long-term success.
For example, bioprinted prosthetics can replicate the intricate structure of bones, allowing for seamless integration with your body. These devices also reduce the risk of complications, such as infections or improper fit. Studies have shown that 3D-printed models align almost perfectly with patient CT scans, with a correlation coefficient of r = 0.9998 and minimal dimensional errors of -0.07 ± 0.35 mm. This precision highlights the effectiveness of bioprinting in creating devices that meet your specific needs.
Anatomical Models for Surgical Planning
Imagine a surgeon practicing your procedure on a model of your exact anatomy before entering the operating room. 3D bioprinting makes this possible by producing highly accurate anatomical models based on your scans. These models help surgeons visualize complex structures, plan their approach, and anticipate challenges.
The accuracy of these models is remarkable. Printing modalities like FDM, SLA, and PolyJet have demonstrated strong positive correlations (r > 0.9) with patient scans, ensuring that the models closely resemble your anatomy. This precision reduces surgical risks and improves outcomes. For you, this means shorter recovery times and fewer complications.
With 3D bioprinting, personalized medicine reaches new heights. Whether it’s custom implants or detailed surgical models, this technology ensures that medical solutions are tailored specifically to you.
Benefits of 3D Bioprinting
Addressing Organ Shortages
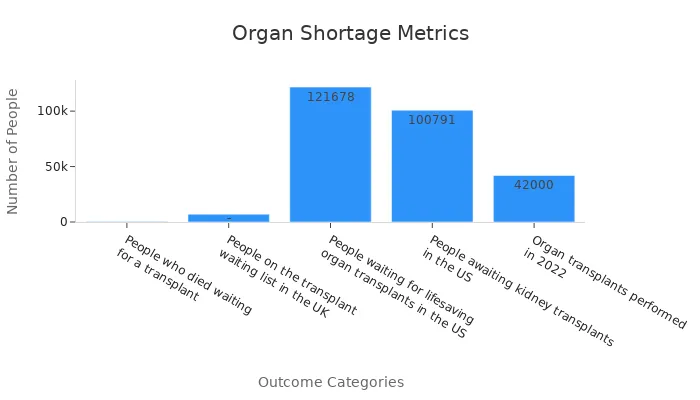
Organ shortages remain a critical issue in healthcare. Every year, thousands of people die while waiting for transplants. In the United States alone, over 121,000 individuals are on the transplant waiting list, with more than 100,000 of them needing kidneys. However, only 42,000 organ transplants were performed in 2022. This gap highlights the urgent need for innovative solutions.
3D bioprinting offers hope by enabling the creation of functional organs. Using your cells, scientists can bioprint organs like kidneys or livers that match your biology. This approach eliminates the dependency on donors and reduces waiting times. The ultimate goal is to scale up the production of living tissues and organs, ensuring that no one has to wait for life-saving transplants.
Statistic Description | Value |
|---|---|
People who died waiting for a transplant | 420 |
People on the transplant waiting list in the UK | 7,000 |
People waiting for lifesaving organ transplants in the US | |
Organ transplants performed in 2022 | 42,000 |
Enhancing Treatment Precision
Precision is vital in medicine, especially when dealing with complex conditions. 3D bioprinting enhances treatment accuracy by creating structures tailored to your anatomy. For example, bioprinted tissues and organs can replicate the exact shape and function of your natural ones. This precision improves the success rate of surgeries and therapies.
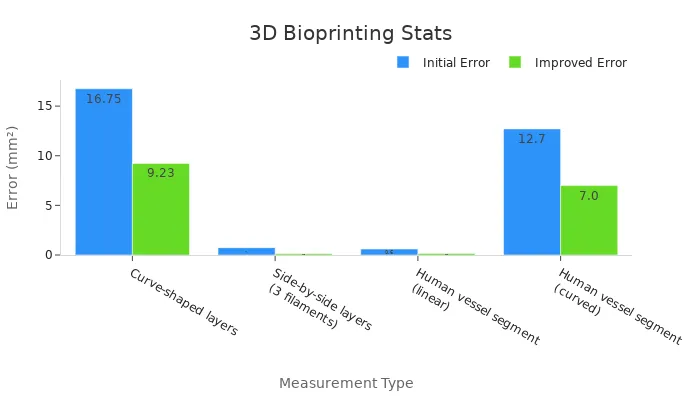
Statistical improvements in bioprinting accuracy demonstrate its effectiveness. For instance, errors in creating human vessel segments have decreased significantly. Linear segments now show an error reduction from 0.6 mm² to just 0.15 mm². Similarly, curved vessel segments have improved from 12.7 mm² to 7.0 mm². These advancements ensure that bioprinted structures closely match your biological needs.
Measurement Type | Initial Error (mm²) | Improved Error (mm²) |
|---|---|---|
Curve-shaped layers | 16.75 | 9.23 |
Side-by-side layers (3 filaments) | 0.74 | 0.13 |
Human vessel segment (linear) | 0.6 | 0.15 |
Human vessel segment (curved) | 12.7 | 7.0 |
Reducing Transplant Rejection Risks
Transplant rejection poses a significant challenge for patients. Your immune system often attacks foreign organs, leading to complications. To prevent this, patients must take immunosuppressive drugs for life, which can cause side effects.
3D bioprinting addresses this issue by creating personalized organs using your genetic material. This customization minimizes the risk of rejection because the bioprinted organ is biologically identical to your own tissues. As a result, you may no longer need lifelong medications, improving your quality of life.
"By using your cells, 3D bioprinting creates organs that your body recognizes as its own, reducing rejection risks and enhancing transplant success."
This revolutionary approach not only improves outcomes but also makes transplants safer and more accessible for patients worldwide.
Accelerating Drug Development
3D bioprinting is revolutionizing drug development by significantly reducing the time and cost involved. Traditional drug development takes nearly 14 years and costs approximately $1.336 billion. This lengthy process often delays the availability of life-saving treatments. With 3D bioprinting, you can accelerate this timeline by creating realistic tissue models that mimic human biology. These models allow researchers to test drugs more efficiently, reducing the need for animal testing and improving accuracy.
Here are some key statistics that highlight the impact of 3D bioprinting on drug development:
The market for 3D bioprinting in drug testing and screening was valued at $580 million in 2020 and is projected to reach $1.9 billion by 2027.
The organ-on-chip market, which relies on 3D bioprinting, was valued at $18.8 million in 2020 and is expected to grow at a CAGR of 37.6% from 2021 to 2028.
By using bioprinted tissues, you can predict how a drug will interact with human cells, reducing the risk of adverse effects. This approach not only speeds up the development process but also ensures that new treatments are safer and more effective. Imagine a future where groundbreaking therapies reach patients in a fraction of the time it takes today. That future is becoming a reality, thanks to 3D bioprinting.
Improving Surgical Outcomes
3D bioprinting is transforming surgeries by enhancing precision and reducing risks. When surgeons use bioprinted anatomical models, they can plan procedures with greater accuracy. These models replicate your unique anatomy, allowing surgeons to anticipate challenges and refine their techniques before the actual operation.
The benefits of 3D bioprinting in surgery are supported by measurable improvements:
Metric | Result |
|---|---|
Operation Time | Significant reduction in operation time observed in case-control studies |
Intraoperative Blood Loss | Clinically significant improvements noted in blood loss metrics |
Postoperative Complications | Reduced complications (OR = 0.42, 95% CI 0.22–0.78) |
Neer Score | Higher Neer score (WMD = 9.57; p < 0.05) |
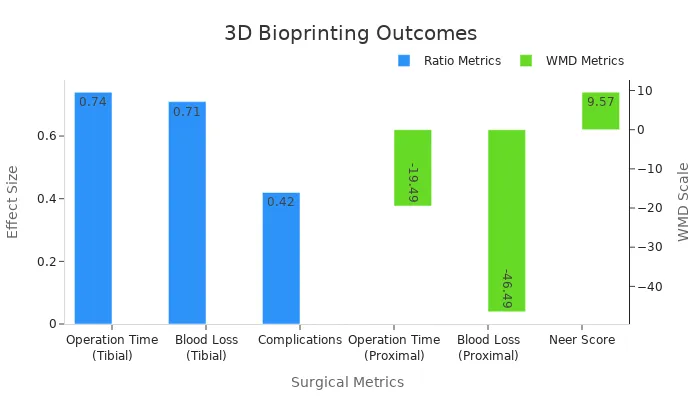
For example, studies show that 3D bioprinting reduces operation time and blood loss during tibial surgeries. Patients experience fewer complications and faster recoveries. By using this technology, you benefit from shorter procedures, reduced risks, and improved outcomes. This innovation ensures that surgeries are safer and more effective, giving you the best possible care.
Challenges and Limitations
Technical and Scientific Hurdles
Functional Viability of Bioprinted Structures
Creating bioprinted structures that function like natural tissues remains a significant challenge. You might wonder why this is so complex. The issue lies in replicating the intricate details of human tissues, such as vascular networks. Without proper blood vessels, tissues cannot survive beyond 100–200 micrometers in thickness. Current printing technologies struggle to fabricate small capillaries, which are essential for transporting nutrients and oxygen. This limitation affects the viability of larger, more complex tissues.
Material and Bioink Constraints
The materials used in 3D bioprinting, known as bioinks, also present hurdles. These materials must mimic the extracellular matrix (ECM) of human tissues to ensure biocompatibility and structural integrity. However, current bioinks fall short of fully replicating native ECM compositions. Additionally, sourcing the large number of cells required for bioprinting is another obstacle. While stem cells show promise, their differentiation into specific cell types is a complex process influenced by multiple factors.
Challenge/Limitations | Description |
|---|---|
Biomaterials | Current printable materials do not fully mimic native ECM compositions, affecting structural integrity and biocompatibility. |
Cell sourcing | Requires a large number of cells, with stem cells being a promising source, but differentiation is influenced at multiple stages. |
Low throughput and high costs | Manual cell seeding and bioink loading are required, leading to low throughput; production costs are high due to expensive reagents. |
Complexity of vascular networks | The ability to print a hierarchical vascular network is crucial for tissue survival, as tissues cannot grow beyond 100–200 μm without adequate vasculature. Current printing resolution limits the fabrication of small blood capillaries. |
Ethical and Regulatory Issues
Ethical Considerations in Bioprinting
The ethical implications of 3D bioprinting are profound. You might ask, "Is it ethical to create functional human organs or tissues?" This question raises concerns about the long-term impact of such advancements. For example, the ability to bioprint organs could lead to debates about human enhancement or the commodification of body parts. Addressing these concerns requires collaboration among scientists, ethicists, and policymakers to ensure responsible use of the technology.
Regulatory Approval Processes
Regulatory agencies play a crucial role in ensuring the safety and quality of bioprinted products. Guidelines for bioinks and bioprinted tissues are essential to maintain high standards. However, translating bioprinting technologies into clinical applications involves rigorous approval processes. These processes aim to address concerns about safety, long-term stability, and ethical implications. Without clear regulations, the path to widespread adoption becomes uncertain.
Regulatory agencies have established guidelines for bioprinted tissue products and bioink materials to ensure safety and quality.
Ethical and regulatory concerns require collaboration among scientists, ethicists, and regulatory bodies.
Clinical applications raise questions about safety, stability, and the ethics of creating functional human tissues.
Cost and Accessibility Challenges
The high cost of 3D bioprinting limits its accessibility. Producing bioprinted tissues involves expensive reagents and manual processes, which reduce throughput and increase costs. For many healthcare systems, these expenses make the technology unattainable. You might wonder how this affects patients. Unfortunately, the financial barriers mean that only a small percentage of people can benefit from these advancements. Reducing costs and improving scalability will be crucial for making 3D bioprinting accessible to all.
The Future of 3D Bioprinting in Medicine
Emerging Trends and Innovations
Advances in Bioink Technology
Bioink technology continues to evolve, offering new possibilities for 3D bioprinting. Researchers are developing bioinks that closely mimic the extracellular matrix of human tissues, improving cell viability and functionality. These advancements allow you to benefit from more accurate tissue models for research and therapy. For example, bioprinted organoids now replicate human organs with remarkable precision. This innovation supports disease studies and drug development, enabling faster and more effective treatments.
The market reflects this progress. The 3D bioprinting industry is projected to grow from $1.6 billion in 2022 to $6.9 billion by 2032, with a compound annual growth rate (CAGR) of 16.1%. Tissue engineering, a key area of bioink application, is expected to expand from $12 billion to $35 billion during the same period. These trends highlight the transformative potential of bioink technology in healthcare.
AI and Machine Learning Integration
Artificial intelligence (AI) and machine learning (ML) are revolutionizing 3D bioprinting. These technologies enhance precision by analyzing vast datasets and optimizing printing parameters. For instance, AI can predict how cells will behave in specific bioinks, ensuring better outcomes. ML algorithms also improve the design of complex structures like vascular networks, which are essential for tissue survival.
The surge in innovation supports this integration. Between 2015 and 2023, the number of patents related to 3D bioprinting reached 2,868, with 24 new companies entering the field last year. This growth demonstrates the increasing role of AI and ML in advancing bioprinting technologies.
Expanding Applications
Rare Disease Treatments
3D bioprinting offers hope for rare disease treatments. By creating patient-specific tissues, you can receive therapies tailored to your unique needs. This approach accelerates the development of treatments for conditions that previously lacked effective solutions. High-throughput capabilities also enable researchers to test multiple therapies simultaneously, speeding up the discovery process.
Customizing Prosthetics and Implants
Personalized prosthetics and implants are becoming more accessible thanks to 3D bioprinting. Using your medical scans, scientists can design devices that fit perfectly, improving comfort and functionality. This customization reduces complications and enhances long-term success. With a projected market size of $6.9 billion by 2032, the potential for personalized medical devices continues to grow.
Long-Term Potential
Overcoming Current Limitations
The future of 3D bioprinting lies in overcoming its current challenges. Researchers are working to improve vascularization in bioprinted tissues, ensuring their long-term viability. Advances in bioink formulations and printing techniques will address these issues, making complex structures like organs more functional.
Transforming Global Healthcare
3D bioprinting has the potential to revolutionize global healthcare. By addressing organ shortages and improving treatment precision, this technology can save countless lives. The precision medicine market, driven by bioprinting, is expected to grow from $83.4 billion in 2022 to $254 billion by 2032. This growth underscores the transformative impact of 3D bioprinting on healthcare systems worldwide.
3D bioprinting is reshaping healthcare by offering solutions tailored to your unique needs. It addresses critical issues like organ shortages and improves treatment precision. You benefit from faster drug development and safer surgeries, thanks to this groundbreaking technology.
"While challenges like cost and scalability remain, the potential of 3D bioprinting to revolutionize medicine is undeniable."
As innovations continue, this technology promises to transform global healthcare and improve patient outcomes. You stand at the forefront of a medical revolution that could redefine the future of personalized medicine.
FAQ
What is the difference between 3D printing and 3D bioprinting?
3D printing uses materials like plastics or metals to create objects. 3D bioprinting, on the other hand, uses living cells and bioinks to create tissues or organs. While both rely on layering techniques, bioprinting focuses on replicating biological structures for medical purposes.
How long does it take to bioprint an organ?
The time depends on the organ's complexity. Simple tissues may take hours, while complex organs like kidneys can take weeks. Researchers also need additional time for testing and maturation to ensure the organ functions properly.
Are bioprinted organs available for transplants today?
Not yet. Scientists are still perfecting the technology to ensure bioprinted organs function like natural ones. Current applications focus on research, drug testing, and simpler structures like skin or cartilage. Full organ transplants may become possible in the future.
Is 3D bioprinting safe for patients?
Yes, but only under strict conditions. Bioprinting uses your cells to reduce rejection risks. However, safety depends on rigorous testing and regulatory approval. Researchers work to ensure bioprinted tissues meet medical standards before clinical use.
Can 3D bioprinting help with rare diseases?
Absolutely! 3D bioprinting creates patient-specific tissues, allowing researchers to study rare diseases more effectively. This personalized approach accelerates the development of treatments tailored to your unique needs, offering hope for conditions that lack effective therapies.
See Also
Key Characteristics and Insights About Hemangioblastoma
Essential Features and Insights Into Glioblastoma
Simplifying the Causes of Gastrointestinal Stromal Tumors
A Simple Guide to B-Cell Prolymphocytic Leukemia
Exploring the Molecular Characteristics of Lung Giant Cell Carcinoma

