What Is Acute Biphenotypic Leukemia and How Is It Defined
Acute Biphenotypic Leukemia is a rare type of acute leukemia. It affects about 1% to 5% of all cases worldwide. Doctors define this disease by finding blast cells that show markers from both myeloid and lymphoid lineages. These blast cells often express antigens such as CD79a, which point to more than one blood cell type. The diagnosis uses systems like EGIL to measure marker coexpression, with scores helping doctors confirm mixed lineage features.
Key Takeaways
Acute biphenotypic leukemia is a rare blood cancer where cells show markers from both myeloid and lymphoid types, making it different from other leukemias.
Doctors diagnose this leukemia using tests like flow cytometry to find specific markers, and they use scoring systems to confirm the mixed cell types.
Children under 14 are most affected, with boys slightly more at risk than girls, and early symptoms include fatigue, bruising, fever, and swollen lymph nodes.
Treatment combines chemotherapy targeting both cell types and often includes stem cell transplantation to improve survival chances.
Early diagnosis and specialized care improve outcomes, and new therapies like immune treatments offer hope for better future results.
Acute Biphenotypic Leukemia Definition
Mixed Lineage Features
Doctors recognize acute biphenotypic leukemia by the presence of blast cells that show both myeloid and lymphoid markers. These mixed lineage features set it apart from other types of leukemia, such as acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), which usually show markers from only one cell type. In acute biphenotypic leukemia, the abnormal cells may express antigens like CD79a for B-lymphoid lineage and CD13 or CD117 for myeloid lineage. This co-expression means the leukemia comes from a stem cell that can turn into more than one type of blood cell.
Mixed lineage features are important because they help doctors identify a rare group of leukemias that need special treatment. These features also mean the disease can be harder to treat and may have a poorer outlook than typical AML or ALL.
The most common marker combinations in acute biphenotypic leukemia are:
Myeloid and B-lineage markers (seen in almost half to three-quarters of cases)
Myeloid and T-lineage markers (less common)
Rarely, all three lineages (myeloid, B, and T) show up together
Doctors use special tests, like flow cytometry, to look for these markers. They do not rely on cell shape alone because the blasts often do not look very different from other leukemias. Instead, they use scoring systems to confirm that the cells truly have features from more than one lineage.
WHO Classification
The World Health Organization (WHO) uses the term "mixed phenotype acute leukemia" (MPAL) to describe acute biphenotypic leukemia. The WHO classification has changed over the years to make the diagnosis more accurate and to separate MPAL from other leukemias with similar features.
The WHO system focuses on a few key points:
Blasts must show strong markers from more than one lineage, not just a few cross-lineage antigens.
Myeloid lineage is confirmed by myeloperoxidase (MPO) positivity.
T-lineage is confirmed by cytoplasmic or surface CD3.
B-lineage is confirmed by strong CD19 plus at least one other B-cell marker (CD79a, cytoplasmic CD22, or CD10).
The WHO also recognizes certain genetic changes, like BCR-ABL1 and KMT2A (MLL) rearrangements, as special subtypes of MPAL. The classification does not set strict cut-off values for marker positivity, so each laboratory may interpret results slightly differently.
Here is a table comparing the main features of the EGIL and WHO classification systems:
Aspect | EGIL Classification System | WHO Classification System |
|---|---|---|
Basis | Scoring system for lineage-specific markers | Uses EGIL scoring within a broader diagnostic approach |
Diagnostic Criteria | Score > 2 for at least two lineages | Includes morphology, cytochemistry, immunophenotyping, genetics |
Marker Panel | Wide panel, weighted by specificity | Emphasizes broad panel, including cytoplasmic markers |
Complexity | Detailed and comprehensive | Stresses comprehensive diagnostic workup |
Clinical Implication | Distinguishes true biphenotypic leukemia | Adds prognostic and clinical relevance |
Recommendation | Standard for diagnosis | Recommends EGIL plus additional modalities |
Over the past decade, the WHO has refined its criteria to improve accuracy. The 2016 update made the rules stricter and included more genetic information. This helps doctors make better decisions about treatment and prognosis for people with acute biphenotypic leukemia.
Diagnosis
Immunophenotyping
Doctors use immunophenotyping as the main tool to diagnose acute biphenotypic leukemia. This test checks for special proteins, called markers, on the surface or inside of leukemia cells. These markers help doctors find out which type of blood cell the leukemia started from. Flow cytometry is the most common method for this test. It can measure many markers at once on thousands of cells.
The most important markers for acute biphenotypic leukemia include:
B-cell markers: CD19, CD79a, CD10
T-cell marker: cytoplasmic CD3
Doctors look for cells that show markers from both myeloid and lymphoid lineages. This co-expression is a key sign of mixed phenotype acute leukemia. The EGIL scoring system helps doctors decide if the cells truly belong to more than one lineage. A score higher than 2 points for two different lineages confirms the diagnosis.
Flow cytometry gives quick and accurate results. It helps doctors see if the leukemia cells have the right combination of markers. This step is very important because it guides the next steps in treatment.
Here is a table showing how some markers are scored in the EGIL system:
Marker | Myeloid Lineage Score | Lymphoid Lineage Score |
|---|---|---|
MPO | 2 | 0 |
CD13 | 1 | 0 |
CD33 | 1 | 0 |
CD19 | 0 | 2 |
CD10 | 0 | 1 |
TdT | 0 | 1 |
Doctors also use genetic tests, such as FISH, to look for changes like KMT2A rearrangements. These tests add more information and help confirm the diagnosis.
Diagnostic Criteria
Doctors follow strict rules to diagnose acute biphenotypic leukemia. The World Health Organization (WHO) calls this disease mixed phenotype acute leukemia (MPAL). The diagnosis depends on finding blast cells that show strong markers from both myeloid and lymphoid lineages.
The main steps in the diagnostic process include:
Doctors check if the leukemia cells look like normal early blood cells from the myeloid, B-cell, or T-cell lineages.
They use flow cytometry to test for key markers. Immunohistochemistry and enzyme cytochemistry can also help.
The EGIL system gives points for each marker. A score above 2 for more than one lineage means the leukemia is biphenotypic. Doctors count a marker as positive if at least 20% of the blasts show it on the surface or 10% inside the cell.
The WHO criteria focus on certain markers: CD19 for B-cells, cytoplasmic CD3 for T-cells, and MPO for myeloid cells. The WHO does not set strict cut-off values but relies on expert judgment.
Doctors must rule out other types of leukemia that only show a few markers from another lineage. Only true cases of mixed phenotype acute leukemia meet the criteria.
Aspect | Details |
|---|---|
Disease Definition | Acute biphenotypic leukemia, now called MPAL, is defined by the presence of both myeloid and lymphoid markers. |
Blasts must show at least 20% positivity for surface markers and 10% for cytoplasmic markers. Key markers include MPO, cytoplasmic CD3, and strong CD19 with other B-cell markers. | |
Frequency | MPAL makes up about 0.5% to 1% of all acute leukemia cases. |
Classification | The WHO excludes cases that do not truly meet biphenotypic criteria. |
Doctors use these criteria to make sure they diagnose only true cases of acute biphenotypic leukemia. This careful process helps guide treatment and gives patients the best chance for a good outcome.
Causes and Risk Factors
Cell Origin
Acute biphenotypic leukemia can start from more than one type of early blood cell. Scientists once thought this disease only came from multipotent hematopoietic stem cells, which can turn into any blood cell. New research shows that early T-cell progenitors, especially at the CD4/CD8 double-negative 2 (DN2) stage, can also give rise to this leukemia. When certain oncogenes, like Myc/Bcl2 or MLL-AF9, affect these DN2 cells, they can develop into leukemia cells with both myeloid and lymphoid features. These DN2-derived cells keep some T-cell memory markers, such as Bcl11b and Gata3, and show a high ability to change into different cell types. Transcription factors like PU.1 help guide this process. This discovery means that acute biphenotypic leukemia can come from several different starting points in the blood cell development pathway.
Genetic changes play a big role in the disease. For example, the MLL-AF9 fusion gene can cause leukemia, but the outcome depends on the cytokine environment. Cytokines like IL-3, IL-7, and FLT3L influence whether the abnormal cells become myeloid, lymphoid, or biphenotypic. In rare cases, families have shown a pattern of inheritance, with both a mother and son developing the disease and sharing certain genetic variants. This suggests that both genetic mutations and the environment inside the bone marrow can increase the risk.
Who Is Affected
Acute biphenotypic leukemia most often affects children. Clinical studies show that most patients are under 14 years old, with a median age of 10. Cases have been reported in infants as young as 7 months and in children up to almost 14 years old. This leukemia is much less common in adults. In children, genetic changes seen in older patients, such as the Philadelphia chromosome, are rare.
A slight difference exists between males and females. Data show that males have a higher incidence rate than females.
Sex | Incidence Rate (per 1,000,000 person-years) |
|---|---|
Male | |
Female | 0.26 |
Acute biphenotypic leukemia remains rare, but understanding who is most at risk helps doctors watch for early signs and improve care for young patients.
Symptoms and Prognosis
Common Symptoms
Acute biphenotypic leukemia often causes a range of symptoms. These symptoms result from both bone marrow failure and the spread of leukemia cells to other tissues. Many patients experience symptoms that overlap with other types of leukemia.
Symptom Category | Symptoms |
|---|---|
Bruising, spotting, anemia, persistent fever, infections, diffuse hemorrhage | |
Symptoms caused by blood cancer cell infiltration into tissues | Swollen lymph nodes, joint pain, swollen gums, enlargement of liver and spleen, headache, vomiting, skin lumps, pericardial or pleural effusion |
Patients may notice the following signs:
Fatigue
Weakness
Lightheadedness or dizziness
Shortness of breath
Easy bruising
Fever
Night sweats
Frequent infections
Enlarged lymph nodes
Swollen gums
Joint pain
Headaches
Nausea
Vomiting
Enlarged liver and spleen
Loss of appetite
Doctors recommend seeking medical attention if these symptoms appear, especially in children or young adults.
Prognosis
The outlook for acute biphenotypic leukemia remains challenging. Clinical studies show that about half of patients achieve complete remission after initial chemotherapy. However, the median survival time is often less than one year, and relapse rates remain high. Patients treated with regimens designed for acute lymphoblastic leukemia (ALL) tend to have higher remission rates than those treated with acute myeloid leukemia (AML) protocols. Median overall survival ranges from 12 to 16 months, with three-year survival rates around 28% to 32%. Children have better outcomes, with five-year survival rates between 36% and 54%, though these rates are still lower than those for standard ALL.
Stem cell transplantation can improve long-term survival. A recent study found a three-year survival rate of about 56% for patients who received a transplant. Children who undergo transplantation may reach a five-year survival rate of 77%.
Several factors influence prognosis:
Younger age, especially under 15 years, improves outcomes.
The presence of the Philadelphia chromosome or complex genetic changes worsens prognosis.
High white blood cell count at diagnosis and certain gene mutations also predict poorer outcomes.
Advances in diagnosis and treatment continue to improve survival, but acute biphenotypic leukemia remains a serious disease that requires specialized care.
Treatment
Treatment Options
Doctors treat acute biphenotypic leukemia with a combination of therapies. They often use chemotherapy regimens that target both myeloid and lymphoid cells. Common protocols include the 7+3 regimen, which uses cytarabine for seven days and an anthracycline for three days. Other regimens, such as VCEPM and the 1423 KFSH protocol, combine drugs like vincristine, cyclophosphamide, etoposide, prednisone, daunorubicin, and L-asparaginase. These treatments aim to destroy leukemia cells from both lineages.
Doctors may also use ALL-type therapies, which have shown good results in some cases. Hybrid regimens that mix drugs for AML and ALL can improve response rates. Newer options, such as venetoclax with hypomethylating agents, are being studied for their effectiveness.
Stem cell transplantation plays a key role in treatment. Many patients receive a transplant after achieving remission. This procedure helps replace diseased bone marrow with healthy cells.
Patients face several side effects during treatment:
Low blood cell counts, which can cause anemia, bleeding, and infections
Tumor lysis syndrome, a condition from rapid cell destruction
Graft versus host disease after stem cell transplantation
Doctors monitor patients closely and use medicines like allopurinol to prevent complications.
Outlook
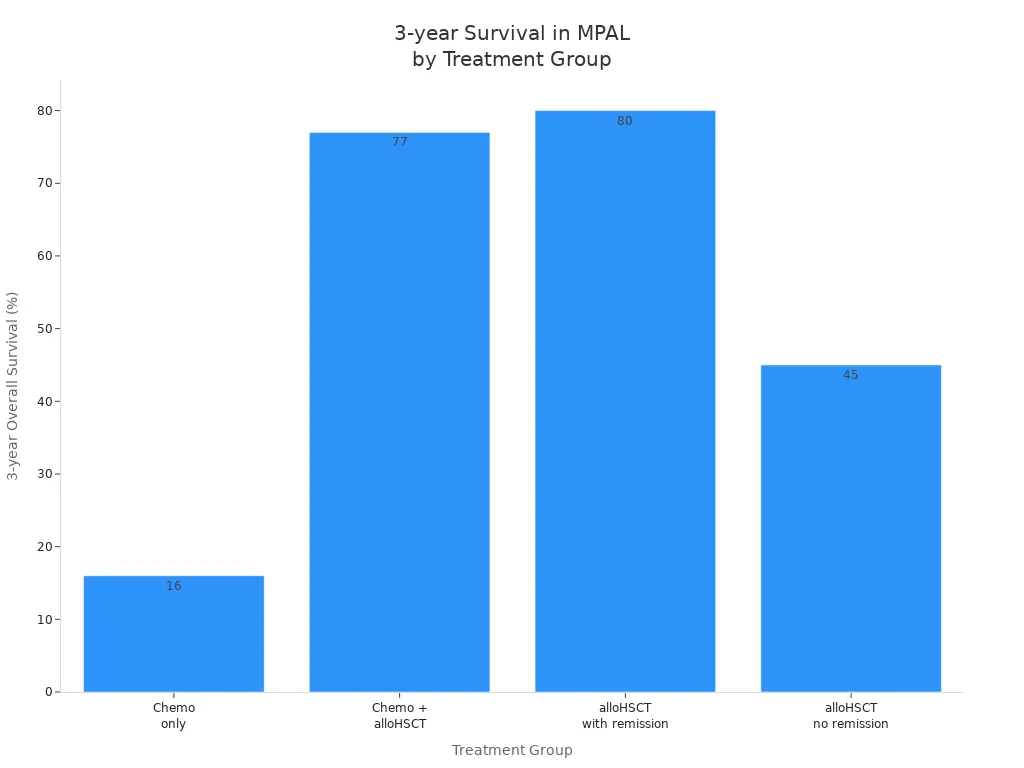
The outlook for acute biphenotypic leukemia depends on the treatment approach. Survival rates improve when doctors use stem cell transplantation after chemotherapy. Patients who receive only chemotherapy have a lower chance of long-term survival.
Treatment Group | Additional Notes | |
|---|---|---|
Chemotherapy only | 16% | Poorer survival compared to alloHSCT |
Chemotherapy + alloHSCT | 77% | Significant survival advantage over chemotherapy |
alloHSCT with remission | 80% | Better outcomes if remission achieved before HSCT |
alloHSCT without remission | 45% | Lower survival if not in remission before HSCT |
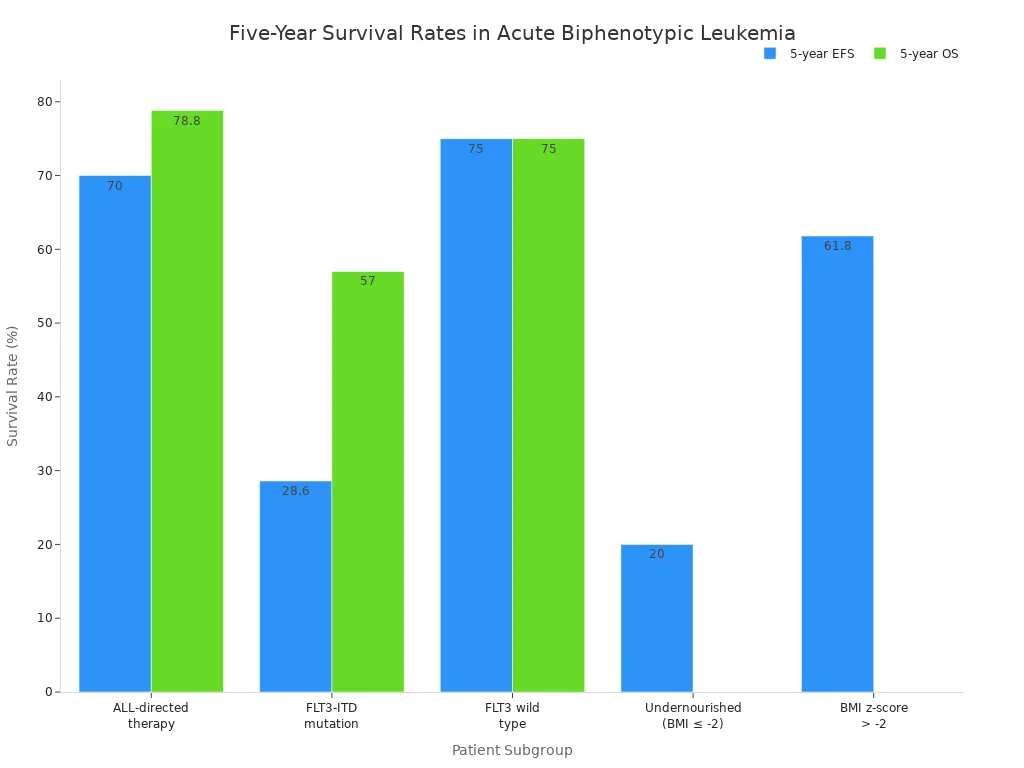
Children treated with ALL-directed therapy show higher five-year survival rates. Event-free survival reaches about 70%, and overall survival approaches 79%. Factors such as age, genetic mutations, and nutritional status affect these outcomes.
Doctors continue to study new treatments to improve survival and reduce side effects. Early diagnosis and specialized care remain important for better outcomes.
Acute Biphenotypic Leukemia stands out because blasts show both lymphoid and myeloid features. Doctors use detailed tests and scoring systems to confirm the diagnosis and rule out similar diseases.
Diagnosis relies on markers like MPO, CD19, and CD3, with the WHO grouping these cases under mixed phenotype acute leukemia.
Patients benefit from early diagnosis and specialized treatment plans.
Recent Advances | Details |
|---|---|
Immune therapies | CAR-T cells, antibody-drug conjugates, and vaccines offer new hope. |
Support | Patients find help through social networks, emotional support, and practical advice. |
Ongoing research and strong support networks help patients and families face this rare disease with hope.
FAQ
What makes acute biphenotypic leukemia different from other leukemias?
Doctors find that acute biphenotypic leukemia has blast cells showing both myeloid and lymphoid markers. Most other leukemias show markers from only one cell type.
How do doctors diagnose acute biphenotypic leukemia?
Doctors use flow cytometry to check for specific markers on leukemia cells. They look for strong evidence of both myeloid and lymphoid features. Genetic tests help confirm the diagnosis.
Who is most likely to get acute biphenotypic leukemia?
Children, especially those under 14 years old, have the highest risk. Males show a slightly higher incidence than females. Adults rarely develop this disease.
Tip: Early medical attention helps improve outcomes for children with symptoms.
What treatments do patients receive for acute biphenotypic leukemia?
Doctors use chemotherapy that targets both cell types. Stem cell transplantation often follows chemotherapy. New treatments, such as immune therapies, continue to improve survival rates.
This article is for educational purposes only and is not a substitute for professional medical advice. For more details, please see our Disclaimer. To understand how we create and review our content, please see our Editorial Policy.
ℹ️ Explore more: Read our Comprehensive Guide to All Known Cancer Types for symptoms, causes, and treatments.
This article is for educational purposes only and is not a substitute for professional medical advice. For more details, please see our Disclaimer. To understand how we create and review our content, please see our Editorial Policy.
#BanishCancer
See Also
Breaking Down B-Cell Prolymphocytic Leukemia Easily
An Introduction To Chronic Lymphocytic Leukemia

