Emerging Trends in Pediatric Oncology Treatments for 2025
Advancements in pediatric oncology treatments for 2025 promise transformative changes in cancer care for children, particularly in the context of 'Conventional Therapies in Pediatric Oncology: What's Changing?'. Researchers have reported significant progress in precision medicine, with functional precision medicine (FPM) approaches improving progression-free survival rates for 83% of pediatric cancer patients in a recent study published in Nature. These innovations aim to reduce the long-term health effects of conventional therapies while addressing disparities in care. For example, nearly 100 hospitals across Latin America and Spain have implemented pediatric early warning systems to lower childhood cancer mortality rates. By refining conventional therapies in pediatric oncology, these advancements offer hope for better survival outcomes and enhanced quality of life for young patients.
Key Takeaways
Precision medicine helps 83% of kids with cancer get better, giving hope for improved treatments.
Early warning systems in hospitals lower death rates in kids with cancer and improve care.
CAR T-cell therapy shows high success for tough cancers in kids, but side effects need watching.
Liquid biopsies are a simple way to check treatments, making it easier for young patients.
Teamwork among doctors, scientists, and leaders is key to better cancer care and fair access to new treatments.
Current Challenges in Pediatric Oncology
Long-Term Health Effects of Conventional Therapies
Conventional pediatric cancer treatments, such as chemotherapy and radiation, have saved countless lives. However, they often leave survivors with significant long-term health issues. Research shows that over 30% of childhood cancer survivors face complications like secondary cancers, cardiovascular diseases, and developmental delays. These late effects can severely impact their quality of life. Survivors may also experience cognitive deficits, which hinder their ability to perform daily tasks or succeed academically.
A study by Bhakta et al. (2017) highlights the cumulative burden of surviving childhood cancer, emphasizing the need for improved survivorship care. Similarly, Suh et al. (2020) provides evidence of chronic health conditions in long-term survivors, further underscoring the importance of refining conventional therapies in pediatric oncology to reduce these risks.
Study | Findings |
|---|---|
Cunningham et al. (2018) | Major causes of death in survivors linked to long-term treatment effects. |
Bhakta et al. (2017) | Significant long-term health effects in childhood cancer survivors. |
Suh et al. (2020) | Chronic health conditions and late mortality in survivors. |
Gaps in Care Transitions for Survivors
Transitioning from active treatment to survivorship care presents numerous challenges. Many survivors do not receive the recommended follow-up care, which is essential for monitoring and managing late effects. Barriers such as inadequate referral networks, lack of caregiver knowledge, and reimbursement issues contribute to these gaps. Families often struggle to access medical and psychosocial support services due to geographical distances, time constraints, and childcare limitations.
Quantitative research highlights these challenges. Families require support services after primary therapy, yet logistical barriers hinder access. Despite these obstacles, parents express satisfaction with available services when they can utilize them. Addressing these gaps is critical to ensuring that survivors receive comprehensive care.
Research Questions | Findings |
|---|---|
Domains of life needing support | Families need support services post-treatment. |
Utilization of follow-up services | Families use medical and psychosocial follow-up services. |
Satisfaction with services | Parents report satisfaction with available services. |
Barriers to access | Distance, time, and childcare issues limit access to services. |
Addressing Disparities in Access to Care
Disparities in pediatric oncology care remain a pressing issue. Survivorship care is often unevenly distributed, with underserved populations facing the greatest challenges. Systemic issues, such as inadequate healthcare infrastructure and limited access to specialized care, exacerbate these disparities. For example, children in rural areas may lack access to pediatric oncologists or survivorship programs, leaving them vulnerable to untreated late effects.
Barriers to care also include financial constraints and a lack of awareness about available resources. Addressing these disparities requires a multifaceted approach, including policy changes, improved referral systems, and increased funding for survivorship programs. By tackling these issues, healthcare providers can ensure equitable access to care for all pediatric cancer survivors.
Conventional Therapies in Pediatric Oncology: What's Changing?
Refining Chemotherapy, Surgery, and Radiation
Conventional therapies like chemotherapy, surgery, and radiation remain the cornerstone of pediatric oncology. However, recent advancements have refined these approaches to improve outcomes and reduce invasiveness. For instance, the FDA's approval of tovorafenib (Ojemda) for children with low-grade glioma has introduced a less invasive treatment option, demonstrating significant tumor shrinkage in clinical trials. Similarly, Lutathera® has emerged as a targeted therapy for pediatric gastroenteropancreatic neuroendocrine tumors, marking a milestone in precision treatment.
Collaborative initiatives such as the Childhood Cancer Data Initiative (CCDI) and the Molecular Characterization Initiative (MCI) are transforming how these therapies are applied. By integrating molecular data into clinical trials, these programs enable more personalized treatment strategies. The Children's Oncology Group (COG), enrolling over 10,000 patients annually, plays a pivotal role in ensuring access to these cutting-edge therapies. These efforts collectively enhance the effectiveness of conventional therapies in pediatric oncology.
Reducing Toxicity and Side Effects
Reducing the toxicity of conventional therapies remains a critical focus in pediatric oncology. High rates of organ toxicity and biochemical abnormalities, such as nephrotoxicity in 18.4% of patients and electrolyte imbalances in 87.5%, highlight the need for safer treatment protocols. Early detection and multidisciplinary approaches have mitigated mortality risks, even in cases of severe toxicity.
Innovative strategies aim to minimize side effects without compromising efficacy. For example, molecular characterization of tumors helps identify somatic aberrations, enabling the development of targeted therapies that reduce the need for high-intensity chemotherapy. These advancements not only improve survival rates but also enhance the quality of life for young patients.
Integrating Liquid Biopsies for Monitoring
Liquid biopsies are revolutionizing how pediatric oncologists monitor treatment efficacy. A 2023 study published in Nature Precision Oncology demonstrated the utility of liquid biopsy tests in assessing treatment responses. These tests have proven effective in differentiating between types of childhood liver cancer and detecting residual brain cancer. This non-invasive approach offers a safer alternative to traditional biopsies, reducing the physical burden on young patients.
By integrating liquid biopsies into routine care, clinicians can make real-time adjustments to treatment plans. This innovation aligns with the broader goal of refining conventional therapies in pediatric oncology, ensuring that treatments are both effective and minimally invasive.
Advancements in Precision Medicine

Genetic and Molecular Profiling
Genetic and molecular profiling has revolutionized pediatric oncology by enabling more precise and effective treatments. Researchers have sequenced 888 pediatric tumors, uncovering genomic variants that align with precision oncology trial protocols. Approximately 33% of patients had at least one variant suitable for these trials, showcasing the practical application of genomic data in treatment planning. This approach not only identifies actionable mutations but also highlights the importance of data sharing to deepen the understanding of pediatric cancer genomics.
Note: Data sharing initiatives play a critical role in advancing molecular profiling, fostering collaboration among researchers, and improving treatment outcomes for young patients.
Tailored Therapies for Specific Cancer Types
Tailored therapies leverage genomic insights to target specific cancer types, offering a personalized approach to treatment. In the same study of 888 pediatric tumors, 14% of patients with actionable variants received molecularly targeted therapy. These therapies demonstrated significant potential to improve outcomes by addressing the unique genetic makeup of each tumor. By integrating these strategies into clinical trials, researchers can maximize the benefits of precision medicine while minimizing the reliance on conventional therapies in pediatric oncology: what's changing is the shift toward more individualized care.
Innovative Technologies in Precision Medicine
Single-Cell Sequencing
Single-cell sequencing has emerged as a groundbreaking tool in pediatric oncology. This technology allows researchers to analyze individual cells within a tumor, uncovering heterogeneity that might otherwise go unnoticed. By identifying specific mutations and cellular behaviors, single-cell sequencing enables the development of highly targeted therapies, improving treatment precision and reducing side effects.
CRISPR and Gene Editing
CRISPR technology has opened new avenues for treating pediatric cancers. By editing faulty genes, CRISPR offers the potential to correct genetic mutations at their source. This approach not only targets cancer cells with unparalleled accuracy but also reduces the need for more invasive treatments. As CRISPR continues to evolve, its integration into pediatric oncology holds immense promise for transforming care.
Breakthroughs in Immunotherapy
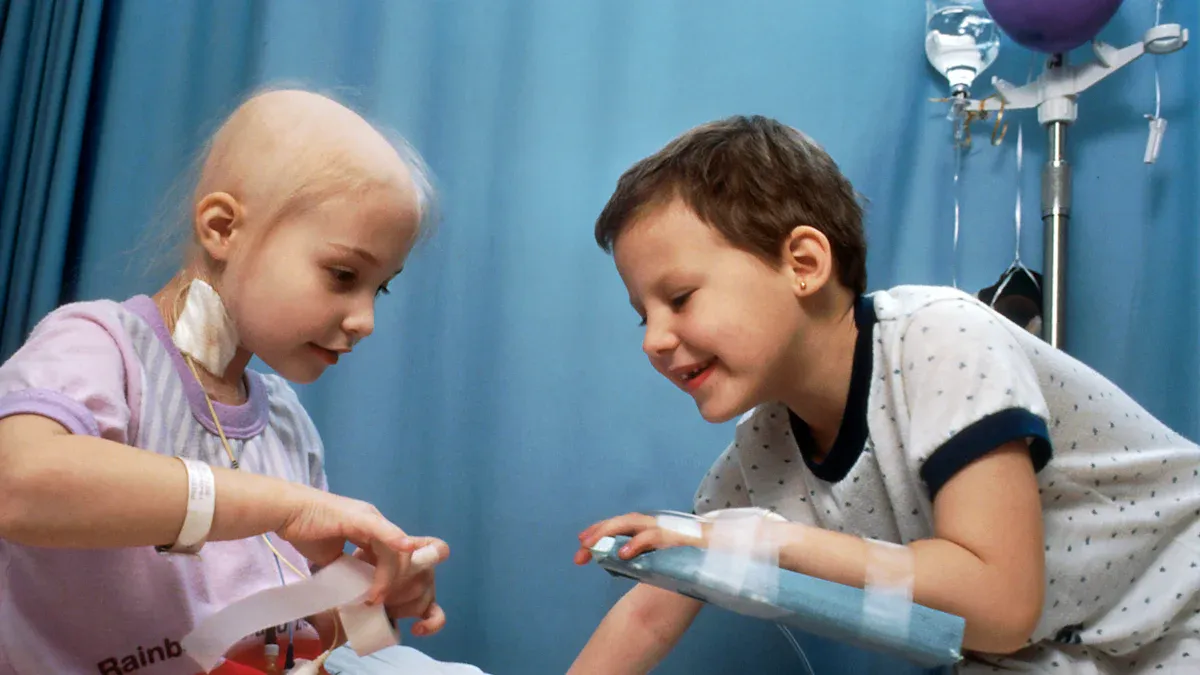
CAR T-Cell Therapies for Pediatric Cancers
CAR T-cell therapy has emerged as a groundbreaking treatment for pediatric cancers, particularly in cases of refractory B-cell acute lymphoblastic leukemia (ALL). This innovative approach involves engineering a patient’s T-cells to target and destroy cancer cells. Clinical trials have demonstrated remarkable remission rates of 70% to 90% in children with refractory B-cell ALL, with some maintaining remission for over six months.
However, challenges remain. Approximately 14% of patients undergoing CAR T-cell therapy experience HLH-like toxicities, a severe inflammatory response. These toxicities significantly impact survival outcomes. Patients with HLH-like toxicities show a one-year relapse-free survival (RFS) rate of 25.7% and an overall survival (OS) rate of 4.7%. In contrast, those without these toxicities achieve much higher rates, with 80.1% RFS and 57.6% OS. Despite these risks, CAR T-cell therapy continues to offer hope for children with limited treatment options.
Cancer Vaccines and Their Potential
Cancer vaccines represent another promising frontier in pediatric oncology. These vaccines aim to stimulate the immune system to recognize and attack cancer cells. Various types of cancer vaccines have shown potential in preclinical and clinical studies:
Vaccine Type | Outcomes | Notes |
|---|---|---|
Dendritic Cell Vaccine | Minimal toxicity, improved outcomes | Tested in neuroblastoma, sarcomas, AML, and brain tumors |
Peptide Vaccines | Complete remission in one patient | Limited patient population, larger studies needed |
Nucleic Acid Vaccines | Induce T-cell immunity | Early studies show potential in training immune response |
Viral Vector Vaccines | Effective in preclinical brain tumor models | Safe with radiation and chemotherapy, stimulates T-cell responses |
These vaccines, though still in development, hold the potential to complement existing therapies and improve survival rates.
Overcoming Scalability Challenges in Immunotherapy
Scaling immunotherapy for widespread use remains a significant hurdle. Manufacturing CAR T-cells is labor-intensive and costly, limiting accessibility. Researchers are exploring automated production systems and off-the-shelf CAR T-cell products to address these challenges. Additionally, global collaborations aim to reduce costs and expand access, ensuring that more children benefit from these life-saving treatments.
The Role of AI in Pediatric Oncology
Enhancing Treatment Personalization
Artificial intelligence (AI) is transforming pediatric oncology by enabling highly personalized treatment plans. AI algorithms analyze vast datasets, including genomic profiles, clinical records, and treatment outcomes, to identify the most effective therapies for individual patients. Molecular profiling of pediatric tumors, for instance, has detected genomic variants in 33% of cases, aligning these patients with precision oncology clinical trials. Similarly, the Pediatric MATCH screening protocol has achieved a 31.5% detection rate, underscoring the potential of AI-driven approaches to refine treatment strategies.
Note: The Molecular Characterization Initiative (MCI) plays a pivotal role in this process. By characterizing the molecular features of pediatric cancers in a CLIA-certified lab, MCI enhances the understanding of genetic factors and supports informed treatment decisions.
AI also facilitates real-time adjustments to treatment plans. Machine learning models predict how tumors will respond to specific therapies, allowing clinicians to modify protocols as needed. This adaptability ensures that young patients receive the most effective and least invasive treatments available.
Predicting Risks and Outcomes
AI excels in predicting risks and outcomes, offering critical insights that guide clinical decision-making. Predictive models analyze patient data to estimate the likelihood of treatment success, recurrence, or adverse effects. These tools help oncologists weigh the benefits and risks of various interventions, ensuring optimal care.
For example, AI systems can identify patterns in imaging data that indicate early signs of relapse. This capability enables timely interventions, improving survival rates. Additionally, AI-driven risk assessments inform families about potential long-term health effects, empowering them to make informed decisions about their child’s care.
Addressing Disparities Through AI-Driven Insights
AI has the potential to bridge disparities in pediatric oncology care. By analyzing data from diverse populations, AI identifies gaps in access to treatment and outcomes. These insights inform policy changes and resource allocation, ensuring equitable care for all patients.
Unordered lists highlight key initiatives addressing disparities:
Molecular Characterization Initiative (MCI): Advances understanding of genetic factors in underserved populations.
AI-Driven Resource Mapping: Identifies regions with limited access to pediatric oncology specialists.
Global Data Sharing Platforms: Promote collaboration and improve care standards worldwide.
AI-driven solutions not only enhance treatment personalization but also ensure that advancements in pediatric oncology benefit every child, regardless of their background.
Collaborative Efforts Driving Progress
Partnerships Between Researchers, Clinicians, and Policymakers
Collaborative partnerships among researchers, clinicians, and policymakers are driving innovation in pediatric oncology. Researchers contribute cutting-edge discoveries, clinicians translate these findings into actionable treatments, and policymakers create frameworks to ensure equitable access. For example, initiatives like the Childhood Cancer Data Initiative (CCDI) foster collaboration by enabling data sharing among specialized cancer centers. This approach accelerates the development of therapies tailored to pediatric patients.
Clinicians play a pivotal role in bridging research and practice. They implement protocols derived from molecular profiling studies, ensuring that treatments align with the unique needs of young patients. Policymakers complement these efforts by funding research programs and establishing guidelines that prioritize pediatric oncology. Together, these partnerships create a cohesive system that advances care and improves outcomes for children battling cancer.
The Role of Advocacy Groups in Advancing Pediatric Oncology
Advocacy groups amplify the voices of pediatric cancer patients and their families. These organizations raise awareness, fund research, and lobby for policy changes that benefit young patients. Their efforts have led to increased funding for pediatric oncology research and the development of survivorship programs.
Groups like the Children’s Oncology Group (COG) exemplify the impact of advocacy. Operating across more than 200 sites globally, COG enrolls over 10,000 patients annually into research protocols. This widespread participation ensures that children from diverse backgrounds benefit from cutting-edge therapies. Advocacy groups also provide educational resources, empowering families to navigate the complexities of cancer care.
Global Initiatives to Improve Access to Cutting-Edge Therapies
Global initiatives are transforming access to advanced pediatric oncology treatments. Programs like the Molecular Characterization Initiative (MCI) have enrolled nearly 4,500 participants, with 66% diagnosed with CNS tumors and 20% with soft tissue sarcomas. By characterizing tumors at a molecular level, MCI enables personalized treatment strategies that improve survival rates.
Unordered lists highlight key achievements:
CCDI enhances access through data sharing among specialized cancer centers.
COG operates across North America, Australia, and New Zealand, enrolling over 10,000 patients annually.
MCI provides molecular profiling for thousands of pediatric cancer patients, advancing precision medicine.
These initiatives demonstrate the power of global collaboration in ensuring equitable access to life-saving therapies for children worldwide.
The advancements in pediatric oncology treatments for 2025 highlight significant progress in improving outcomes for young patients. Key breakthroughs include:
Functional Precision Medicine: This approach, combining genetic and drug testing, improved outcomes for 83% of children with relapsed or refractory cancer.
Pediatric Early Warning System: Implemented in nearly 100 hospitals, this system has reduced childhood cancer mortality rates by enhancing patient care.
Collaboration among researchers, clinicians, and policymakers remains essential to sustaining these advancements. The future of pediatric cancer care looks promising, with innovative therapies and global initiatives paving the way for better survival rates and quality of life.
FAQ
What is the role of precision medicine in pediatric oncology?
Precision medicine uses genetic and molecular profiling to create personalized treatment plans for children with cancer. This approach targets specific mutations in tumors, improving treatment effectiveness and reducing side effects. It represents a significant shift from traditional, one-size-fits-all therapies.
How does CAR T-cell therapy work for pediatric cancers?
CAR T-cell therapy modifies a child’s T-cells to recognize and attack cancer cells. This innovative treatment has shown high remission rates in children with refractory B-cell acute lymphoblastic leukemia. However, it requires careful monitoring due to potential toxicities.
Are liquid biopsies safe for children?
Yes, liquid biopsies are safe and non-invasive. They analyze blood samples to monitor cancer progression or treatment response. This method reduces the need for traditional biopsies, which can be more invasive and painful for young patients.
How does AI improve pediatric cancer care?
AI analyzes large datasets to personalize treatments, predict outcomes, and identify risks. It helps clinicians make data-driven decisions, ensuring children receive the most effective therapies. AI also addresses disparities by mapping underserved areas and improving access to care.
What global initiatives support pediatric oncology advancements?
Programs like the Molecular Characterization Initiative (MCI) and the Childhood Cancer Data Initiative (CCDI) enhance access to cutting-edge treatments. These initiatives promote data sharing, molecular profiling, and collaboration among researchers, ensuring equitable care for children worldwide.
See Also
Understanding Treatment Options for Acute Myeloid Leukemia
An In-Depth Overview of Various Cancer Types
Recognizing Symptoms and Treatments for Duodenal Cancer
Key Characteristics and Explanation of Hemangioblastoma
Identifying Symptoms and Treatment Options for Conjunctival Melanoma

