Dual-Antibody Immunotherapy Shows Promise for Extramedullary Multiple Myeloma
You may face limited options if you have extramedullary multiple myeloma, an aggressive form of cancer. Dual-antibody immunotherapy for multiple myeloma now offers hope. A recent Mayo Clinic study shows this treatment can lead to deep and lasting responses. In clinical studies, some therapies like talquetamab reached response rates above 70% in certain groups, while teclistamab showed lower rates in extramedullary cases.
Key Takeaways
Dual-antibody immunotherapy offers hope for patients with extramedullary multiple myeloma, showing response rates above 70%.
This treatment uses two engineered antibodies to help the immune system target and attack myeloma cells effectively.
Patients can start dual-antibody immunotherapy immediately, without waiting for cell processing, which is crucial for aggressive disease.
The therapy has shown a median duration of response of 13.8 months, providing longer disease control for patients.
Supportive care strategies can help manage side effects and improve quality of life during treatment.
Dual-antibody Immunotherapy for Multiple Myeloma
How the Therapy Works
You may wonder how dual-antibody immunotherapy for multiple myeloma targets cancer cells. This treatment uses two engineered antibodies, talquetamab and teclistamab. These antibodies help your immune system find and attack myeloma cells, even when the disease spreads outside the bone marrow.
Here is a simple table that explains how this therapy works:
Mechanism of Action | Description |
|---|---|
Dual-target strategy | Uses two antibodies that engage T cells through separate immune pathways. |
Targeting myeloma cells | Directs T cells to attack myeloma cells, which is helpful in tough cases. |
Comparison to CAR-T | Delivered as a standard injection at the clinic, so you do not need special cell processing. |
The antibodies in this therapy each have a special job. Some target proteins on the surface of myeloma cells, while others help guide your immune cells to the right place. Here are some of the main targets:
Antibody Target | Role in Therapy |
|---|---|
CD3 | Helps redirect immune cells |
BCMA | Focuses the immune attack on tumors |
GPRC5D | Finds tumor-associated antigens |
CD38 | Finds tumor-associated antigens |
FcRH5 | Finds tumor-associated antigens |
Tip: By using more than one antibody, this therapy can attack the cancer from different angles. This makes it harder for the disease to escape.
Off-the-shelf Benefits
Dual-antibody immunotherapy for multiple myeloma gives you access to treatment right away. Unlike some other therapies, you do not have to wait weeks for your cells to be processed in a lab. Doctors can give you this therapy as soon as you need it.
This approach has shown strong results. In one study, 79% of patients responded to treatment, and 54% had no detectable disease after therapy. These numbers show that dual-antibody immunotherapy for multiple myeloma can work well, even for people with aggressive forms of the disease.
Here is a quick look at how quickly you can get this therapy compared to others:
Therapy Type | Administration Time | Availability |
|---|---|---|
Off-the-shelf Dual-Antibody | Available right away | |
CAR-T Cell Therapy | 3 to 4 weeks (manufacture) | Must wait for custom cells |
Note: Quick access to treatment can make a big difference if your disease is progressing fast.
Comparison to CAR-T and Standard Treatments
You might ask how dual-antibody immunotherapy for multiple myeloma stacks up against other treatments. CAR-T cell therapy and standard treatments like chemotherapy have helped many people, but they also have limits.
The table below compares the main features of these therapies:
Treatment Type | CR Rate (95% CI) | ORR (95% CI) | Adverse Events (CRS) | Severe CRS Rate (Grade ≥3) |
|---|---|---|---|---|
CAR-T Cell Therapy | 0.83 (0.76–0.90) | 0.83 (0.70–0.97) | 0.07 (0.03–0.14) | |
Bispecific Antibodies | 0.35 (0.30–0.41) | 0.65 (0.59–0.71) | 0.59 (0.43–0.74) | 0.01 (0.00–0.02) |
Ciltacabtagene Autoleucel | 0.77 (0.71–0.84) | 0.91 (0.83–0.99) | N/A | N/A |
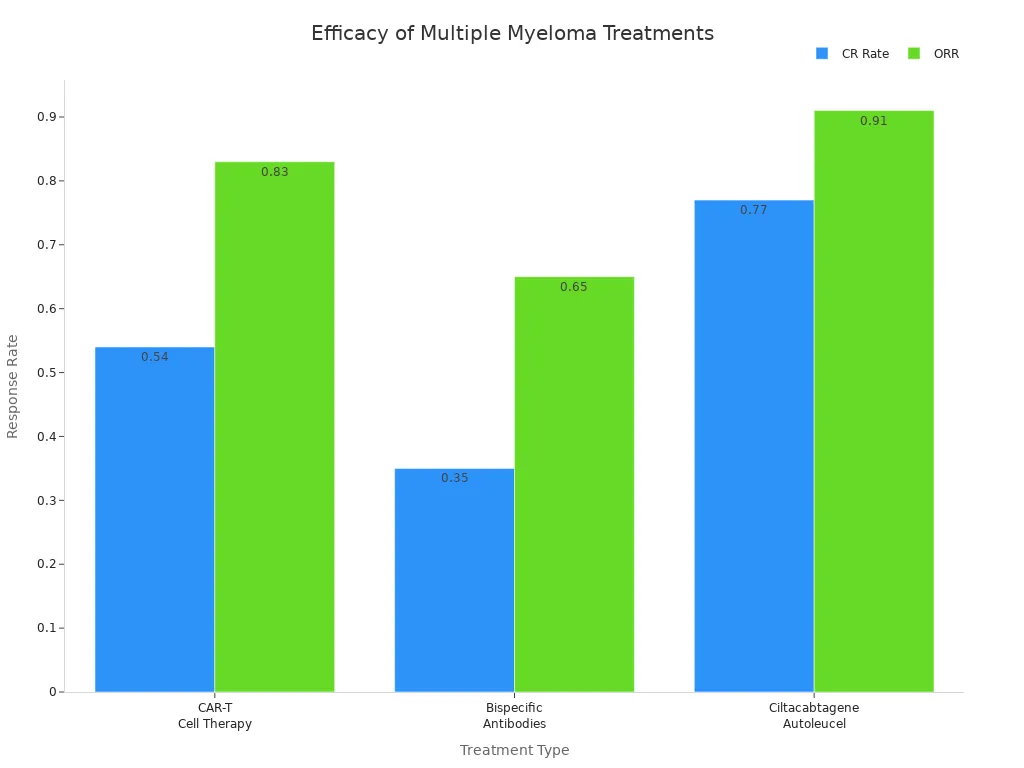
You can see that dual-antibody immunotherapy for multiple myeloma offers strong response rates and fewer severe side effects than CAR-T cell therapy. Most people experience some side effects, but these are often less severe than those seen with CAR-T. You may still need to watch for infections, fatigue, or blood count changes, but the risk of very serious reactions is lower.
Remember: Dual-antibody immunotherapy for multiple myeloma gives you a new option that is both powerful and easier to access than many older treatments.
Clinical Trial Results

Study Design and Patient Group
You may want to know how researchers tested dual-antibody immunotherapy for multiple myeloma in patients with extramedullary disease. The RedirecTT-1 trial used an open-label design in Phase Ib/II. Doctors gave patients a combination of talquetamab and teclistamab. You needed to have tried other treatments before joining this study. These treatments included a proteasome inhibitor, an immunomodulator, and an anti-CD38 monoclonal antibody. You also needed to have extramedullary disease with at least one lesion outside the bone and not treated with radiation. Some patients had nonsecretory or oligosecretory disease, which means their cancer did not produce many abnormal proteins.
Here is a table that shows the main features of the trial:
Trial Name | Phase | Design | Treatment Combination | Patient Selection Criteria |
|---|---|---|---|---|
RedirecTT-1 | Ib/II | Open-label | Talquetamab and Teclistamab | Prior exposure to key drugs; extramedullary disease with at least one non-irradiated, bone-independent lesion; nonsecretory or oligosecretory disease |
Doctors enrolled 158 patients with extramedullary multiple myeloma. Most patients were men, and the median age ranged from 53 to 74 years. Over half had a good performance status, which means you could still do daily activities. Most patients identified as White. The age and treatment history matched those of patients without extramedullary disease.
Characteristic | Value |
|---|---|
Total patients with EMD | 158 |
Male patients (%) | 56% (89 out of 158) |
Median age range | 53 to 74 years |
ECOG performance status (≥ 50%) | 1 |
Self-identified as White (≥ 75%) | Yes |
Comparison with patients without EMD | Similar age and treatment history |
Response and Remission Rates
You may wonder how well dual-antibody immunotherapy for multiple myeloma worked for these patients. The trial showed high response rates. Many patients saw their cancer shrink or disappear. In the study, 79% of patients responded to treatment. Over half of the patients had no detectable disease after therapy. These results are important because extramedullary multiple myeloma often resists standard treatments.
Note: High response rates mean you have a better chance of controlling your disease, even if other treatments have failed.
Durability of Disease Control
You may ask how long the benefits of this therapy last. The trial found that the median duration of response reached 13.8 months. This means that, for most patients, the cancer stayed under control for more than a year after starting treatment.
You can expect a median duration of disease control of 13.8 months with dual-antibody immunotherapy for multiple myeloma.
This long-lasting effect gives you more time without disease progression.
Doctors consider this a major step forward for patients with aggressive forms of multiple myeloma.
😊 If you have extramedullary multiple myeloma, these results show that dual-antibody immunotherapy can offer hope for longer and deeper remission.
Safety and Side Effect Management
Common Side Effects
You may experience side effects when you receive dual-antibody immunotherapy for multiple myeloma. Most patients report some symptoms. About 87% of people in clinical trials had side effects. The most common problems include:
Low white blood cell counts (62%)
Infections (37%)
Cytokine release syndrome (CRS), which happened in 78% of patients, but was usually mild
Neurological effects, such as confusion or headaches, in 12% of patients, with only two severe cases
Nail changes, like brittleness or discoloration, in over half of patients at certain dose levels
Skin issues, such as dryness or itching, in up to 70% of patients
You should tell your doctor about any new symptoms. Early reporting helps your care team manage side effects quickly.
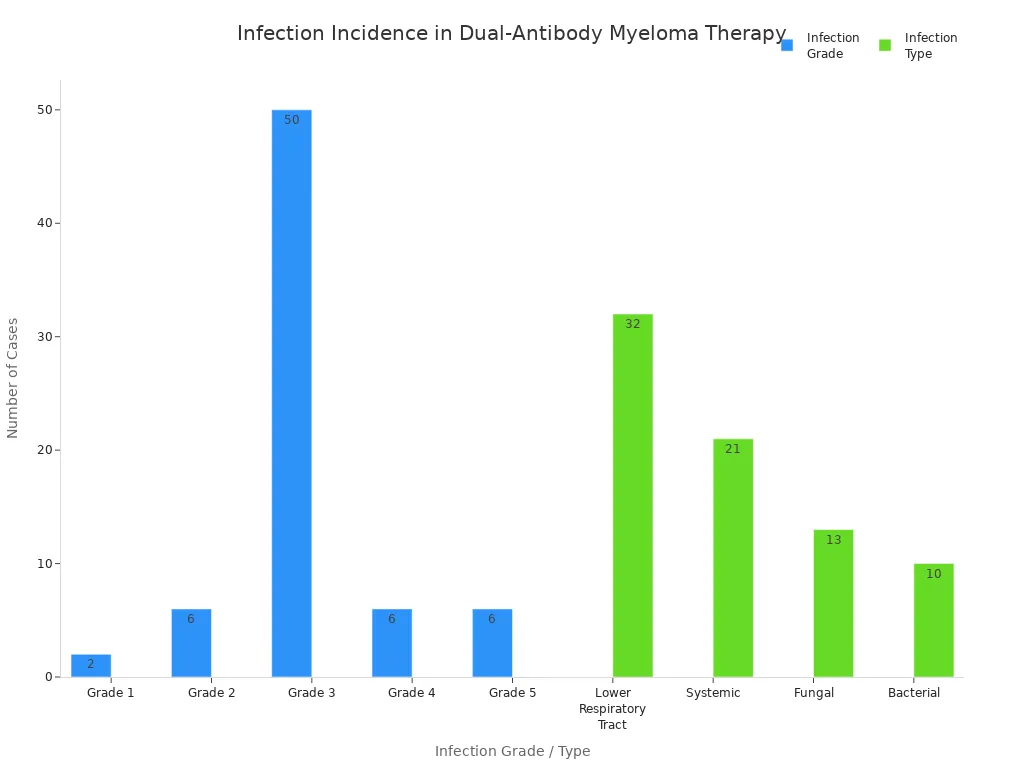
Infection Risks and Prevention
Your risk for infections increases with dual-antibody immunotherapy. Severe infections can happen, especially if your white blood cell count drops. Pneumonia and respiratory infections are common. The chart below shows infection grades and types among patients:
Doctors use several strategies to lower your risk:
Pathogen | Prevention Method | When to Use |
|---|---|---|
Bacterial | Antibiotics (levofloxacin, cefdinir, etc.) | Start with therapy, continue first month |
Viral (Herpes, Varicella) | Acyclovir or valacyclovir | Ongoing |
Fungal | Fluconazole | If white blood cells are low |
Pneumococcus, Influenza, COVID | Vaccines | Before and during therapy |
Hepatitis B | Entecavir or tenofovir | If you test positive |
You should follow your doctor’s advice about vaccines and medicines. These steps help prevent serious infections.
Supportive Care Strategies
Supportive care helps you feel better and stay on treatment longer. Your care team may use medicines, therapies, and lifestyle changes to reduce side effects. Here are some ways supportive care can help:
Supportive care can prevent delays or stopping treatment. It improves your quality of life and helps you get the most benefit from therapy.
Supportive Care Intervention | Benefit |
|---|---|
Bisphosphonates | Less bone pain and fewer fractures |
Vertebroplasty/Kyphoplasty | Better movement and less pain |
Prophylactic antibiotics | Fewer infections |
Plasmapheresis | Less need for dialysis |
Treatment of bone lesions | Improved physical and emotional health |
Immunizations also protect you from common germs. You may not respond as well to vaccines as other people, so your doctor may adjust your schedule. Good symptom control lets you stay on therapy and enjoy a better quality of life.
Future Directions
Earlier Use in Treatment
You may soon see dual-antibody immunotherapy used earlier in your treatment plan. Researchers are testing these therapies in patients who have not yet received many other treatments. For example, a Phase 2 trial uses linvoseltamab, which targets CD3 and BCMA, in patients who still have minimal disease after combination therapy.
Trial Focus | Treatment | Phase | Participants Enrolled |
|---|---|---|---|
Dual-antibody immunotherapy for multiple myeloma | Linvoseltamab | Phase 2 | 25 |
You can look at the response rates from early studies. These numbers show strong results, especially for patients who receive the recommended dose.
Patient Group | Overall Response Rate (ORR) |
|---|---|
All dose levels | 86.6% (71/82) |
Recommended Phase 2 regimen (RP2R) |
High response rates suggest that starting dual-antibody immunotherapy sooner may help you achieve better outcomes.
Application to Other Cancers
You may wonder if dual-antibody immunotherapy can help with other cancers. Researchers are studying these therapies in both blood cancers and solid tumors.
Patients with B-cell non-Hodgkin lymphoma who failed other treatments have achieved complete and lasting remissions with drugs like mosunetuzumab.
Some people whose lymphoma returned after CAR T-cell therapy saw their cancer disappear with dual-antibody immunotherapy.
Studies are ongoing in lung cancer and transformed lymphomas. Early results show improved survival, but also more side effects.
Researchers continue to test these therapies in solid tumors, hoping to expand their use.
Dual-antibody immunotherapy could become an option for many types of cancer in the future.
Improving Safety
You may worry about side effects. Doctors are working on ways to make dual-antibody immunotherapy safer for you. They use guidelines based on clinical experience and collect real-time data to adjust care.
Strategy | Description |
|---|---|
Expert Consensus Guidelines | Doctors share best practices for using bispecific antibodies. |
Real-time Data Collection | Teams track patient outcomes to improve safety during treatment. |
You may also benefit from these supportive measures:
Prophylactic IVIG for low antibody levels
Granulocyte colony-stimulating factor to boost white blood cells
Antiviral and antibacterial drugs to prevent infections
Herpes zoster and PJP prevention for at-risk patients
Doctors use new medicines and lower doses to reduce side effects. They may give glucocorticosteroids for cytokine release syndrome or anakinra if symptoms do not improve. Researchers are testing dual-payload antibody-drug conjugates, which combine different agents to fight cancer and lower toxicity.
You can expect safer treatments as doctors learn more and improve therapy protocols.
You now have more hope if you face extramedullary multiple myeloma. Dual-antibody immunotherapy shows strong results and helps control tough disease. The table below highlights promising treatments:
Treatment Combination | Outcome | Notes |
|---|---|---|
Teclistamab and talquetamab | Promising results | Effective against extramedullary disease |
Bispecific antibodies with daratumumab or isatuximab and pomalidomide | Better outcomes | Improved efficacy in multiple myeloma treatment |
Cevostamab with pomalidomide and dexamethasone | 100% overall response rate | Works best in combination |
Ongoing research brings new hope:
Doctors move bispecific antibodies into earlier treatment lines.
Tecvayli-Darzalex combination improves survival rates and reduces disease progression.
More patients live longer after receiving these therapies.
Experts agree that immunotherapy is changing care for multiple myeloma. You can expect safer treatments and better results as research continues.
FAQ
What is dual-antibody immunotherapy?
You receive two antibodies that help your immune system find and attack myeloma cells. This therapy works even when your cancer spreads outside the bone marrow.
How soon can you start this treatment?
You can start dual-antibody immunotherapy right away. Doctors do not need to custom-make the treatment for you. This quick access helps if your disease progresses fast.
What side effects should you watch for?
You may notice low white blood cell counts, infections, or mild cytokine release syndrome. Some people see skin or nail changes. Tell your doctor about any new symptoms.
Tip: Early reporting of side effects helps your care team keep you safe.
How does this therapy compare to CAR-T cell therapy?
Feature | Dual-Antibody Immunotherapy | CAR-T Cell Therapy |
|---|---|---|
Start Time | Immediate | 3–4 weeks |
Severe Side Effects | Less common | More common |
Customization Needed | No | Yes |
Can you receive vaccines during treatment?
You can get some vaccines, like flu or COVID-19 shots, before or during therapy. Your doctor will guide you on the best timing. Vaccines help protect you from infections.
This article is for educational purposes only and is not a substitute for professional medical advice. For more details, please see our Disclaimer. To understand how we create and review our content, please see our Editorial Policy.
See Also
Exploring Treatment Options For Acute Myeloid Leukemia
Anaplastic Large Cell Lymphoma: Overview And Treatment Approaches
Key Characteristics And Management Of Intravascular Large B-Cell Lymphoma
AIDS Related Lymphoma: Insights And Treatment Strategies
Post-Diagnosis Insights On Angioimmunoblastic T-Cell Lymphoma

