How CRISPR Is Transforming Cancer Gene Editing in 2026
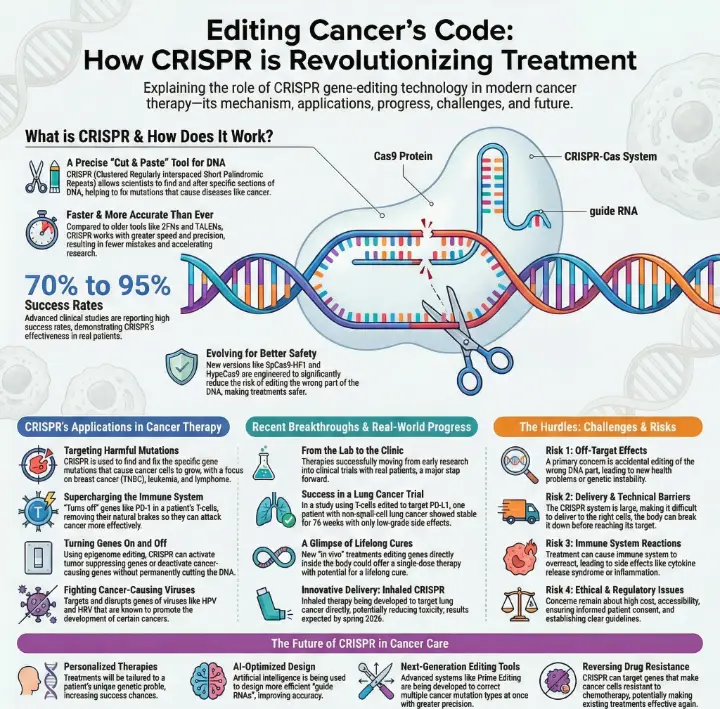
You now see how CRISPR is changing the fight against cancer in 2026. With CRISPR-Cas12, you get gene editing that works faster and with more accuracy than ever. This technology gives you new hope by making treatments that fit your unique needs. You benefit from safer therapies because CRISPR-Cas12 reduces mistakes in your DNA. The Impact of CRISPR Technology on Cancer Gene Editing helps doctors target cancer cells and create better therapies for you.
Key Takeaways
CRISPR-Cas12 offers faster and more accurate gene editing, leading to safer cancer treatments with fewer mistakes.
Researchers are using CRISPR to target harmful mutations in various cancers, improving treatment options for patients.
New CRISPR therapies are moving from trials to real-world applications, providing hope for effective and long-lasting cancer care.
CRISPR can enhance immune cell function, making treatments more effective against cancer by boosting the body's natural defenses.
Ongoing challenges include improving delivery methods and addressing ethical concerns, but advancements in CRISPR technology continue to evolve.
The Impact of CRISPR Technology on Cancer Gene Editing

Precision and Speed in DNA Modification
You now see how the impact of CRISPR technology on cancer gene editing changes the way you fight cancer. CRISPR gives you tools that work with high accuracy and speed. You can trust these tools to make fewer mistakes in your DNA. This means safer treatments for you and better results for doctors.
Nearly 300 clinical trials around the world show how CRISPR helps treat many diseases, including cancer.
Advanced studies report success rates between 70% and 95%. These numbers show how well CRISPR works in real patients.
New versions of Cas9, like SpCas9-HF1, lower the risk of editing the wrong part of your DNA.
CRISPR can reach hard-to-edit spots in your genes, making it useful for many types of cancer.
You benefit from faster research, too. In the past, scientists used tools like ZFNs and TALENs. These tools took a lot of time and money to design. With CRISPR, you can change many genes at once. This lets researchers test ideas quickly and find answers faster. The impact of CRISPR technology on cancer gene editing means you get new treatments sooner.
Note: Pharmacodynamic changes in CRISPR tools also help lower side effects, making your therapy safer.
Understanding Cancer Development with CRISPR
You can use CRISPR to learn how cancer starts and grows. The impact of CRISPR technology on cancer gene editing helps you find and target the mutations that cause cancer. Scientists use CRISPR to study which genes make cancer cells survive or die.
Findings | |
|---|---|
CRISPR/Cas12 for synthetic lethality | Finds gene pairs that, when changed, kill cancer cells but not healthy ones. |
CRISPR/Cas12 in cancer diagnostics | Spots mutations in genes like KRAS, TP53, and BRAF. This helps you catch cancer early. |
CRISPR/Cas13 for oncogene knock-down | Lowers the activity of cancer-causing genes. This can shrink tumors and help you heal. |
You see that the impact of CRISPR technology on cancer gene editing gives you hope for better tests and treatments. You can look forward to more ways to stop cancer before it spreads.
CRISPR Applications in Cancer

Targeting Harmful Mutations
You can use CRISPR to find and fix harmful mutations in cancer cells. This tool helps you target the exact genes that cause cancer to grow. Many scientists focus on cancers like breast cancer, especially triple negative breast cancer (TNBC), leukemia, and lymphoma. These types of cancer respond well to CRISPR-based treatments.
Breast cancer, particularly triple negative breast cancer (TNBC)
Hematological cancers such as leukemia
Lymphoma
You see many clinical and preclinical studies using CRISPR to target these harmful mutations. The table below shows how researchers test CRISPR in different cancers:
Type of Cancer | Number of Trials |
|---|---|
Solid Cancers | At least 3 ongoing trials |
Hematologic Malignancies | Included in overall trials |
You also benefit from new CRISPR-based cell therapies. For example, studies in lung cancer show that these therapies are safe and work well. Other studies report similar results, giving you hope for better treatments.
Study Focus | Findings |
|---|---|
CRISPR-based cell therapy for lung cancer | Clinically feasible and generally safe |
Other recent studies | Echoed similar safety and feasibility conclusions |
You see that most early trials focus on blood cancers, while later trials include more solid tumors.
Trial Phase | Focus Area | Percentage |
|---|---|---|
Phase 1 | Hematologic malignancies | |
Phase 2 | Solid tumors | 46% |
The Impact of CRISPR Technology on Cancer Gene Editing means you get more precise and safer treatments for these cancers.
Gene Activation and Deactivation Strategies
You can use CRISPR not only to cut out harmful genes but also to turn genes on or off. This strategy helps you fight cancer in new ways. For example, CRISPR/Cas9 can change the cancer genome, which is important for treatment. You can boost your immune system by removing genes that slow down T cells, such as CTLA-4 and PD-1. This makes your immune cells stronger against cancer.
CRISPR/Cas9 technology can manipulate the cancer genome, which is essential for cancer treatment.
It enhances T-cell-based immunotherapy by removing genes that inhibit T-cell activity, such as CTLA-4 and PD-1.
The system can target and disrupt genes associated with various viruses that promote cancer, such as HPV and HBV.
CRISPR/Cas9 can be used for epigenome editing, allowing for activation or repression of oncogenes and tumor suppressor genes.
You can also use CRISPR to fight viruses that cause cancer, like HPV and HBV. By turning off these viral genes, you lower your risk of cancer. CRISPR can even change how genes work without cutting DNA. This process, called epigenome editing, lets you turn cancer genes on or off as needed.
RNA-Targeting Platforms and Immune Cell Modulation
You can use new CRISPR tools that target RNA instead of DNA. These tools help you control how your immune cells work. For example, you can improve tumor-infiltrating lymphocytes (TILs), which are immune cells that attack cancer. CRISPR helps you find the genes that make cancer cells survive. You can then change these genes to make your immune cells stronger.
A genome-scale CRISPR-Cas9 mutagenesis screen was used in human melanoma cells to identify genes that enable immune escape from T cell-mediated cytolysis.
The study reported previously undescribed genes and microRNAs that facilitate tumor destruction by T cells.
The correlation of these candidate genes with cytolytic activity was examined in ~11,000 human tumors from The Cancer Genome Atlas (TCGA).
You also see CRISPR used in lung cancer research. Scientists knocked out the NRF2 gene in lung cancer cells. This made the cells grow slower and respond better to chemotherapy. In another study, CRISPR created mutations in the EGFR gene, which helps doctors understand drug resistance.
You benefit from modified Cas9 enzymes, which make CRISPR more accurate. The table below shows how these new enzymes improve your safety:
Cas9 Variant | Improvement in Specificity | Mechanism of Action |
|---|---|---|
SpCas9-HF1 | Reduced off-target mutations | Engineered to minimize non-specific DNA interactions. |
eSpCas9(1.1) | Enhanced precision in genome editing | Exhibits precision genome-editing in human cells with reduced off-target mutations. |
HypaCas9 | Hyper-accurate performance without efficiency loss | Disruption of the REC3 domain enhances target complementarity and catalytic competence. |
You see that CRISPR can also help you overcome drug resistance. By targeting genes linked to resistance, you can make cancer cells more sensitive to treatment. This gives you a better chance to beat cancer.
Tip: You can ask your doctor about new CRISPR-based therapies if you have a cancer that is hard to treat.
CRISPR Breakthroughs in Cancer
Recent Clinical Trials and Successes
You see new clinical trials using CRISPR that change how doctors treat cancer. Researchers now move from laboratory experiments to real patient care. You benefit from therapies that edit genes inside your body. These treatments can work with just one dose and may last a lifetime.
Here are some key milestones that shape CRISPR cancer research:
Scientists shift from lab research to clinical applications.
Doctors use in vivo CRISPR-Cas9 therapies for direct gene editing in patients.
CRISPR helps create better disease models and improves drug development.
You can look at recent trial results to see how CRISPR works in real patients. The table below shows a study using PD-L1 edited T cells for lung cancer:
Trial Focus | Patient Count | Edited T Cells Infused | Safety Report | Objective Response | Notable Outcome |
|---|---|---|---|---|---|
PD-L1 edited T cells for refractory non-small-cell lung cancer | 22 | 12 | Low grade adverse events | None | One patient with higher PD-1 editing efficiency showed stable disease for 76 weeks |
You notice that most patients have mild side effects. One patient with high editing efficiency stays stable for over a year. This gives you hope for safer and longer-lasting treatments.
Real-World Treatments in 2026
You now see CRISPR therapies move from trials to real-world use. Doctors focus on making treatments more effective and easier to deliver. You benefit from new solutions that tackle tough problems like treatment resistance.
Teams combine innovative science with practical delivery methods.
New inhaled CRISPR therapy targets lung cancer directly and reduces toxicity.
Studies on this therapy start soon, with results expected by spring 2026.
You also see a new method that activates genes without cutting DNA. This approach removes chemical tags that silence genes. You may get safer treatments for diseases like Sickle Cell disease.
Note: The revolutionary potential of CRISPR-Cas9 now appears in single-dose therapies that could offer lifelong cures.
You find that CRISPR breakthroughs in 2026 give you more choices and better outcomes. Doctors continue to improve these therapies, making cancer treatment safer and more effective for you.
Challenges of CRISPR in Cancer Therapy
Off-Target Effects and Safety
You may wonder about the risks of CRISPR in cancer therapy. One main concern is off-target effects. These happen when CRISPR edits the wrong part of your DNA. Unintended changes can cause new problems, such as other diseases or genetic instability. You also face risks like immune system overactivation, which can lead to inflammation or even autoimmune reactions. Some patients experience cytokine release syndrome or graft versus host disease. Doctors worry about p53-mediated cell toxicity and the chance of tumors forming after gene editing. The way CRISPR enters your body can also affect your immune response and safety.
Note: Researchers work hard to improve CRISPR’s accuracy and reduce these risks, but safety remains a top priority.
Delivery Methods and Technical Barriers
You need CRISPR to reach the right cells in your body. This step is not easy. The CRISPR system is large, which makes delivery hard. Your body’s fluids can break down CRISPR before it reaches cancer cells. The negative charge and water-loving nature of CRISPR parts make it tough for them to cross cell membranes. Even after entering a cell, CRISPR can get trapped and destroyed in lysosomes or endosomes.
You see scientists using viral and non-viral systems to solve these problems. Viral vectors help target delivery, while non-viral methods like lipid nanoparticles and electroporation offer other options. Each method has limits. For example, electroporation needs special tools and can damage tissues. Nanoparticles and liposomes improve targeting but still face challenges in stability and efficiency.
Delivery Method | Description |
|---|---|
Viral Systems | Use viruses to deliver CRISPR to target cells. |
Non-Viral Systems | Use liposomes, nanoparticles, or electroporation. |
Ethical and Regulatory Issues
You must also think about the ethical side of CRISPR. Weak rules around germline editing raise concerns. Some worry about new mutations or diseases from off-target effects. Not everyone can afford CRISPR therapies, which may increase health gaps. Informed consent is another issue, especially for very sick patients who may not understand all the risks.
Regulatory agencies try to keep up with CRISPR’s fast growth. Many CRISPR cancer drugs are still in early testing. The rules for these treatments are still changing, and experts call for stronger guidelines to protect you and other patients.
Tip: You should always ask your doctor about the risks and benefits of new CRISPR therapies.
Future Outlook for CRISPR Cancer Therapies
Next Steps in CRISPR-Based Treatments
You will see CRISPR technology change cancer care in many ways over the next few years. Experts predict that CRISPR will help doctors treat more types of cancer and create new therapies for tough cases. You may benefit from treatments that use your own immune cells, which scientists can engineer to find and destroy cancer. Doctors also plan to target genes that make cancer cells resist chemotherapy. For example, disabling the NRF2 gene can help restore drug sensitivity.
You might receive therapies designed just for you. These personalized treatments will match your genetic profile, giving you a better chance to beat cancer. Researchers are working on viruses that attack only tumor cells. CRISPR can also edit mutations that make cancer hard to treat, offering new hope for patients who do not respond to standard therapies.
Clinical trials now focus on CRISPR’s power to treat cancer, building on the success of gene editing for other diseases.
What’s coming next?
Engineering immune cells to attack cancer
Targeting genes to reverse drug resistance
Personalized therapies for each patient
Tumor-specific viruses that destroy cancer cells
Editing treatment-resistant mutations
Remaining Hurdles and Opportunities
You still face challenges with CRISPR cancer therapies. Delivery methods must improve so CRISPR can reach the right cells without causing harm. Scientists are developing new genome editing tools, like Prime Editing, which can fix many mutations at once. High-fidelity CRISPR tools now offer better precision, lowering the risk of mistakes.
Advances in artificial intelligence help design guide RNAs, making gene editing more efficient. You may see CRISPR delivered directly into the tumor microenvironment, which increases accuracy and reduces side effects. New systems use Cas-expressing cell lines and stem cells to make treatments easier to produce.
Opportunity | Description |
|---|---|
Prime Editing | Corrects multiple cancer mutations |
AI-optimized guide RNAs | Improves editing efficiency |
Tumor-specific delivery | Targets cancer cells, reduces off-target effects |
You will benefit from these breakthroughs as scientists overcome technical barriers. The future of CRISPR in cancer therapy looks bright, with safer and more effective treatments on the horizon.
You see CRISPR changing cancer gene editing in big ways.
CRISPR helps you understand cancer better and makes immune cells stronger for treatment.
You notice new therapies for brain, kidney, and colon cancers.
Safety and delivery remain tough problems.
Ethical questions about gene editing and access still matter.
Stay curious about CRISPR news. You may find hope in future breakthroughs. Keep learning and support research for better cancer care. 🚀
FAQ
What is CRISPR?
You use CRISPR as a tool to change genes. Scientists use it to cut or fix DNA in cells. This helps you fight diseases like cancer. CRISPR stands for "Clustered Regularly Interspaced Short Palindromic Repeats."
Is CRISPR safe for cancer treatment?
You see CRISPR as a safe tool in many studies. Doctors work hard to lower risks. You may still face side effects, but new versions of CRISPR make treatments safer every year.
Tip: Always talk to your doctor about the risks and benefits.
How does CRISPR help your immune system fight cancer?
You can use CRISPR to make your immune cells stronger. Scientists edit genes in T cells. These cells then find and attack cancer better. You get a better chance to heal.
Can you get CRISPR cancer therapy now?
You may join clinical trials if you qualify. Most CRISPR cancer therapies are still in testing. You should ask your doctor about new trials or approved treatments near you.
Therapy Status | Availability |
|---|---|
Clinical Trials | Limited |
Approved Treatments | Few, expanding |
This article is for educational purposes only and is not a substitute for professional medical advice. For more details, please see our Disclaimer. To understand how we create and review our content, please see our Editorial Policy.
See Also
An In-Depth Overview of Various Cancer Types
Understanding Choriocarcinoma: Symptoms and Treatment Options
Key Characteristics of Glioblastoma: A Detailed Explanation

