Breakthrough combination therapies offer hope for MECOM-rearranged AML patients
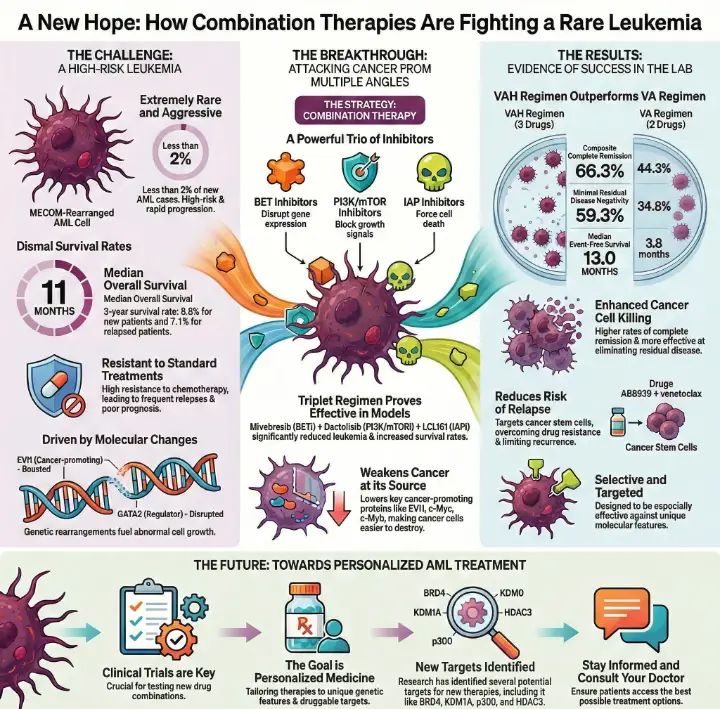
Patients face a tough battle when diagnosed with MECOM-rearranged acute myeloid leukemia. This rare form of leukemia appears in less than 2% of new AML cases and causes severe challenges for doctors and families. Survival rates remain low, with most patients living only 5.9 to 8.4 months after diagnosis. Recent studies reveal that MECOM-rearranged acute myeloid leukemia combination therapies show promise in overcoming resistance and improving outcomes for those affected.
Key Takeaways
MECOM-rearranged AML is a rare and aggressive leukemia subtype, with patients facing rapid disease progression and limited treatment options.
Combination therapies using targeted inhibitors can effectively attack cancer cells from multiple angles, improving survival rates for MECOM-rearranged AML patients.
Ongoing clinical trials are crucial for discovering new treatments and understanding which therapies work best for individual patients.
Personalized treatment approaches can enhance outcomes by matching therapies to the unique genetic features of each patient's disease.
Staying informed about new developments and consulting healthcare providers can help patients access the best treatment options available.
Why MECOM-rearranged AML Is Challenging
Aggressive Disease Features
MECOM-rearranged AML stands out as one of the most aggressive forms of leukemia. Patients often face rapid disease progression and limited treatment options. Doctors classify this subtype as high-risk because it resists standard chemotherapy and relapses frequently. The prognosis remains poor compared to other AML subtypes. The table below highlights key clinical differences:
Feature | MECOM-Rearranged AML | Other AML Subtypes |
|---|---|---|
Classification | High-risk subtype | Varies (can be low, intermediate, high) |
Chemotherapy Resistance | High resistance to standard chemotherapy | Varies (some subtypes respond well) |
Relapse Rates | Higher relapse rates | Generally lower in favorable subtypes |
Prognosis | Poor overall prognosis | Better prognosis in favorable cases |
Treatment Response | Often requires alternative treatment approaches | More predictable responses in some subtypes |
Survival statistics reinforce the severity of this disease:
The median overall survival for patients with MECOM-rearranged AML is 11 months.
The 3-year overall survival rate for newly diagnosed patients is 8.8%.
The 3-year overall survival rate for relapsed or refractory patients is 7.1%.
EVI1 and GATA2 Molecular Changes
Researchers have identified unique molecular changes in MECOM-rearranged AML. The EVI1 gene plays a critical role in the development of certain AML subtypes. Genetic rearrangements at the MECOM locus disrupt the expression of important regulators like GATA2. These changes lead to abnormal cell growth and therapy resistance. The table below explains how EVI1 and GATA2 contribute to disease mechanisms:
Evidence Description | Explanation |
|---|---|
EVI1 is critical for the pathogenesis of a subset of MLL-AF9-rearranged AMLs. | EVI1 drives disease development in specific AML subtypes. |
3q26.2 rearrangements targeting the MECOM locus abrogate expression of MDS1-EVI1 and other myeloid regulators. | Genetic changes affect key regulators, including GATA2. |
The repositioning of a GATA2 distal hematopoietic enhancer into the vicinity of EVI1 homologs. | Enhancer hijacking alters gene expression patterns. |
The G2DHE element in 3q-rearranged AML is a monoallelic super-enhancer formed on the oncogenic EVI1 allele. | Overexpression of EVI1 impacts GATA2 levels. |
The interplay between deregulated EVI1 and disturbances in GATA2 dosage is linked to therapy-resistant AML. | These molecular changes contribute to resistance. |
Resistance to Standard Treatments
Patients with MECOM-rearranged AML often do not respond well to conventional therapies. The RUNX1::MECOM fusion and the EVI1 oncogene drive resistance to chemotherapy. Doctors observe poor outcomes and frequent relapses. These challenges highlight the need for new approaches, such as MECOM-rearranged acute myeloid leukemia combination therapies, which aim to overcome resistance and improve survival.
MECOM-rearranged Acute Myeloid Leukemia Combination Therapies
Targeted Inhibitors: BET, PI3K/mTOR, and IAP
Researchers at MD Anderson Cancer Center have identified a powerful strategy for treating MECOM-rearranged acute myeloid leukemia. They use a combination of targeted inhibitors that block key survival pathways in cancer cells. The three main types include BET inhibitors, PI3K/mTOR inhibitors, and IAP inhibitors. Each drug targets a different weakness in the leukemia cells:
BET inhibitors disrupt proteins like BRD4, which control gene expression.
PI3K/mTOR inhibitors block signals that help cancer cells grow and survive.
IAP inhibitors prevent cancer cells from avoiding programmed cell death.
Evidence from Preclinical Research
Scientists found that combining these inhibitors leads to better results than using them alone. In laboratory and animal studies, the combination of mivebresib (BET inhibitor), dactolisib (PI3K/mTOR inhibitor), and LCL161 (IAP inhibitor) significantly reduced leukemia burden and increased survival rates in mice with MECOM-rearranged AML. This triplet regimen works by lowering levels of cancer-promoting proteins such as EVI1, c-Myc, and c-Myb, which are typically overexpressed in this aggressive subtype.
Key 2026 Context
Clinical Potential: This study provides the "rational basis" for moving into human clinical trials for these specific triplet regimens in 2026.
Survival Impact: Historically, this subtype has a less-than-10% five-year survival rate, making these new combination findings highly significant for patients with limited options.
The rationale for using MECOM-rearranged acute myeloid leukemia combination therapies comes from understanding how EVI1 activates the PI3K/mTOR pathway, which causes resistance to treatment. The table below summarizes the scientific basis for this approach:
Evidence Type | Description |
|---|---|
Mechanism | Activation of the PI3K/mTOR pathway by EVI1 linked to therapy resistance in MECOM-r AML cells. |
Efficacy | BET, PI3K/mTOR, and IAP inhibitors show superior in vitro and in vivo efficacy against MECOM-r AML cells. |
Dependencies | Identified dependencies include BRD4, PIK3CA, mTOR, BCL-xL, and XIAP in MECOM-r AML cells. |
Treatment Outcomes | Combination therapies induce greater lethality in MECOM-r AML cells compared to monotherapies. |
Survival | In a MECOM-r AML PDX model, combination therapies significantly reduced AML burden and increased survival. |
Mechanisms: Blocking Survival Pathways
MECOM-rearranged acute myeloid leukemia combination therapies work by attacking the cancer cells from multiple angles. BET inhibitors stop the cells from making proteins that help them grow. PI3K/mTOR inhibitors block signals that tell the cells to survive and divide. IAP inhibitors force the cells to undergo apoptosis, which is a natural process of cell death.
The combination of these drugs disrupts several survival pathways at once. This makes it harder for the leukemia cells to adapt or become resistant. The following table shows how these treatments affect AML burden and survival rates:
Treatment | Effect on AML Burden | Survival Rate | Statistical Significance |
|---|---|---|---|
Combination therapies targeting specific dependencies | Increased survival | p < 0.05 | |
ORY001 (LSD1i) | Moderate, dose-dependent cell death | Induced differentiation | N/A |
mivebresib (BETi) + dactolisib (mTOR/PI3Ki) | Reduced viability in PD AML samples | Greater effect in 3q26.2-r AML | p < 0.01 |
In preclinical models, the combination of these inhibitors led to a dramatic decrease in leukemia cells and improved survival.
The therapies showed greater effectiveness in MECOM-rearranged AML compared to other subtypes.
Lowering Cancer-Promoting Proteins
MECOM-rearranged acute myeloid leukemia combination therapies also lower the levels of proteins that help cancer cells survive. These proteins include EVI1, c-Myc, and c-Myb. By reducing these proteins, the therapies weaken the cancer cells and make them easier to destroy.
The table below highlights the impact of combination therapies on cancer-promoting proteins:
Cancer-Promoting Protein | Effect of Combination Therapies |
|---|---|
EVI1 | Reduced levels |
c-Myc | Reduced levels |
c-Myb | Reduced levels |
Overall Efficacy | Significant improvement in killing cancer cells and increasing survival rates in lab models |
Doctors and scientists believe that lowering these proteins is a key reason why MECOM-rearranged acute myeloid leukemia combination therapies work so well. The approach attacks the disease at its source and prevents the cancer cells from escaping treatment.
Breakthrough Results of Combination Therapies

Enhanced Cell Killing in Lab Models
Researchers observed remarkable improvements in cell killing when they tested new drug combinations in laboratory models. Scientists compared different treatment regimens and measured how many leukemia cells survived. The VAH regimen, which combines homoharringtonine, venetoclax, and azacitidine, produced higher rates of complete remission and minimal residual disease negativity than the VA regimen. The following table shows the results:
Treatment Regimen | Composite CR Rate | MRD Negativity | Median EFS (months) | Median OS (months) |
|---|---|---|---|---|
VAH (Homoharringtonine + Venetoclax + Azacitidine) | 59.3% | 13.0 | Not reached | |
VA (Venetoclax + Azacitidine) | 44.3% | 34.8% | 3.8 | 15.1 |
These findings suggest that combining multiple targeted agents can destroy more leukemia cells and help patients achieve deeper remissions.
Selectivity for MECOM-rearranged AML
MECOM-rearranged acute myeloid leukemia combination therapies show strong selectivity for this aggressive subtype. Scientists designed triplet regimens to target the unique molecular features of MECOM-rearranged AML. Studies revealed that these combinations act more effectively against MECOM-rearranged cells than other AML types. The table below highlights this selectivity:
Treatment Regimen | Key Findings | Implications |
|---|---|---|
Triplet regimen | Selective activity against MECOM-rearranged AML | Highlights the role of mechanism-driven combination strategies in aggressive cancers |
This selectivity means that patients with MECOM-rearranged AML may benefit more from these therapies than those with other forms of leukemia.
Improved Survival and Reduced Resistance
Combination therapies not only kill more cancer cells but also help prevent drug resistance. Scientists tested regimens like AB8939 plus venetoclax, which destabilize microtubules and target cancer stem cells. These combinations reduced resistance and limited the risk of relapse. The table below summarizes the impact:
Drug Combination | Mechanism of Action | Impact on Resistance |
|---|---|---|
AB8939 + Venetoclax | Pro-apoptotic, destabilizes microtubules, targets cancer stem cells | Reduces resistance and limits relapse risk |
Researchers also noted that therapy resistance and poor outcomes remain major challenges in MECOM-rearranged AML. The need for combination therapies is clear:
Study Focus | Outcome | Relevance |
|---|---|---|
MECOM-rearranged AML | Therapy resistance and poor outcomes | Supports the need for combination therapies to improve treatment efficacy |
These results demonstrate that combining targeted agents can improve survival and reduce the chance of relapse for patients with MECOM-rearranged AML.
Future Outlook for Patients
Clinical Trial Implications
Ongoing research and clinical trials play a vital role in advancing treatment for MECOM-rearranged AML. Scientists continue to test new drug combinations in both laboratory and patient settings. These trials help doctors understand which therapies work best and which patients benefit the most. Clinical trials also ensure that new treatments are safe and effective before they become widely available. Patients who join these studies may gain early access to promising therapies and contribute to medical progress.
Toward Personalized AML Treatment
Researchers now focus on tailoring treatments to each patient's unique disease features. By identifying specific genetic changes and druggable targets, doctors can select therapies that match the biology of each case. Recent studies show that combination therapies can reduce leukemia burden and increase survival in animal models. Scientists have found that targeting proteins like BRD4, KDM1A, p300, and HDAC3 can improve outcomes. The table below highlights key findings that support a personalized approach:
Evidence Description | Findings | Implications |
|---|---|---|
Combination therapies reduced in vivo AML burden | In a luciferized 3q26.2-r AML PDX model, combinations reduced AML burden and increased survival compared to single agents (p < 0.05) | Supports the efficacy of combination therapies in personalized treatment for MECOM-rearranged AML patients |
Identification of druggable targets | CRISPR and chemical screens identified BRD4, KDM1A, p300, and HDAC3 as dependencies | Highlights potential targets for combination therapies in MECOM-rearranged AML |
Induction of differentiation and apoptosis | Treatments with LSD1i, HDAC3i, and CBP/p300i induced cell death and differentiation in 3q26.2-r AML cell lines | Suggests that combination therapies can enhance treatment efficacy by targeting multiple pathways |
Next Steps in Research
Researchers plan to expand clinical trials and explore new drug combinations. They aim to discover more about how these therapies work and how to predict which patients will respond best. Scientists also want to find ways to prevent resistance and relapse. As knowledge grows, doctors hope to make personalized treatment the standard of care for MECOM-rearranged AML. Patients and families can look forward to more options and better outcomes in the future.
Breakthrough combination therapies offer new hope for MECOM-rearranged AML patients. These treatments target cancer cells more effectively and may improve survival. Continued research and clinical trials remain essential for advancing care.
Ongoing cytogenetic and molecular analysis helps doctors track changes during relapse.
Prompt intervention with tailored therapies improves outcomes, especially for patients with complex genetic profiles.
Staying informed about new developments and consulting healthcare providers ensures patients receive the best possible options.
FAQ
What makes MECOM-rearranged AML different from other types of leukemia?
Doctors recognize MECOM-rearranged AML as a rare and aggressive subtype. Patients often experience rapid disease progression and resistance to standard treatments. Unique genetic changes drive this form of leukemia.
How do combination therapies improve outcomes for MECOM-rearranged AML patients?
Researchers found that using multiple targeted drugs attacks cancer cells from several directions. These therapies block survival signals and lower cancer-promoting proteins. Patients may see better remission rates and longer survival.
Are MECOM-rearranged acute myeloid leukemia combination therapies available for all patients?
Many therapies remain in clinical trials. Doctors may recommend participation in studies for eligible patients. Availability depends on location and trial enrollment.
What side effects can patients expect from these new therapies?
Patients may experience fatigue, nausea, or low blood counts. Doctors monitor side effects closely and adjust treatment as needed. Each patient responds differently.
How can families stay informed about new treatment options?
Families should talk with healthcare providers regularly.
They can follow updates from cancer research organizations.
Joining support groups helps families share experiences and learn about new therapies.
This article is for educational purposes only and is not a substitute for professional medical advice. For more details, please see our Disclaimer. To understand how we create and review our content, please see our Editorial Policy.
See Also
Treatment Approaches for Acute Myeloid Dendritic Cell Leukemia
Anaplastic Large Cell Lymphoma: Definition and Treatment Options
LGL Leukemia: Important Symptoms, Diagnosis, and Treatment Insights
Intravascular Large B-Cell Lymphoma: Key Characteristics Explained
Large Cell Lung Carcinoma with Rhabdoid Features: An Overview

