How Cancer Cell Stickiness Could Predict Breast Cancer Spread

Measuring how sticky cancer cells are may help predict if breast cancer will spread. Scientists have found that breast cancer cells with weak adhesion often cause more lung metastases than those that stick strongly. A microfluidic device for cancer cell adhesion can sort tumor cells by their stickiness, helping doctors spot which tumors might spread. Up to 61% of ductal carcinoma in situ cases may be low risk and overtreated, so a better test could help patients avoid unnecessary surgery or therapy.
Key Takeaways
Measuring how sticky cancer cells are helps predict if breast cancer will spread to other parts of the body.
A new microfluidic device sorts cancer cells by stickiness, allowing doctors to identify high-risk tumors quickly and accurately.
Weakly sticky cancer cells are more likely to break away and cause metastasis, while strongly sticky cells tend to stay put.
This technology can reduce overtreatment by helping doctors decide which patients need aggressive care and which can avoid unnecessary surgery or therapy.
Future research aims to improve these tests and apply them to other cancers, offering hope for better personalized treatment and early detection.
Predicting Cancer Spread
Adhesion and Metastasis
Cancer cells do not stay in one place. They can break away from the original tumor and travel to other parts of the body. This process is called metastasis. Scientists have learned that cell adhesion, or how sticky a cell is, plays a big role in this process. When cancer cells lose their stickiness, they can detach more easily from the main tumor. These less adhesive cells can then move through the body and start new tumors in places like the lungs or liver.
Recent research shows that cell adhesion molecules help cancer cells interact with their surroundings. These molecules let tumor cells attach to other cells and to the extracellular matrix, which is a network that supports tissues. When adhesion is weak, cancer cells can invade nearby tissues and enter the bloodstream. Once in the blood, they can travel to distant organs. Molecules like integrins and focal adhesion kinase help control how cells stick and move. Changes in these molecules can make cancer cells more likely to spread.
A new microfluidic device can measure how sticky cancer cells are. This device uses chambers coated with fibronectin, a protein found in the body, and tests how well cells hold on under flowing liquid. Studies in mice and human tissue samples show that cells with low adhesion are more likely to cause metastasis. This finding could help doctors predict which tumors are most dangerous.
Current Limitations
Doctors use several tools to predict if breast cancer will spread. Imaging methods like MRI and CT scans help find tumors in bones, lungs, or liver. Machine learning models and genetic tests also give clues about risk. However, these methods have some problems:
Many models use small groups of patients, so results may not apply to everyone.
Some tests group different types of metastasis together, making it hard to know the exact risk for each organ.
Most genetic tests work best for certain breast cancer types, like ER-positive tumors, and may not help with others.
There are few tests that can spot metastasis early, before it becomes a big problem.
Doctors still rely on their own judgment because no single tool is perfect.
Note: New methods like the microfluidic device offer hope, but they need more testing in larger and more diverse patient groups to become part of regular care.
Cell Adhesion Basics
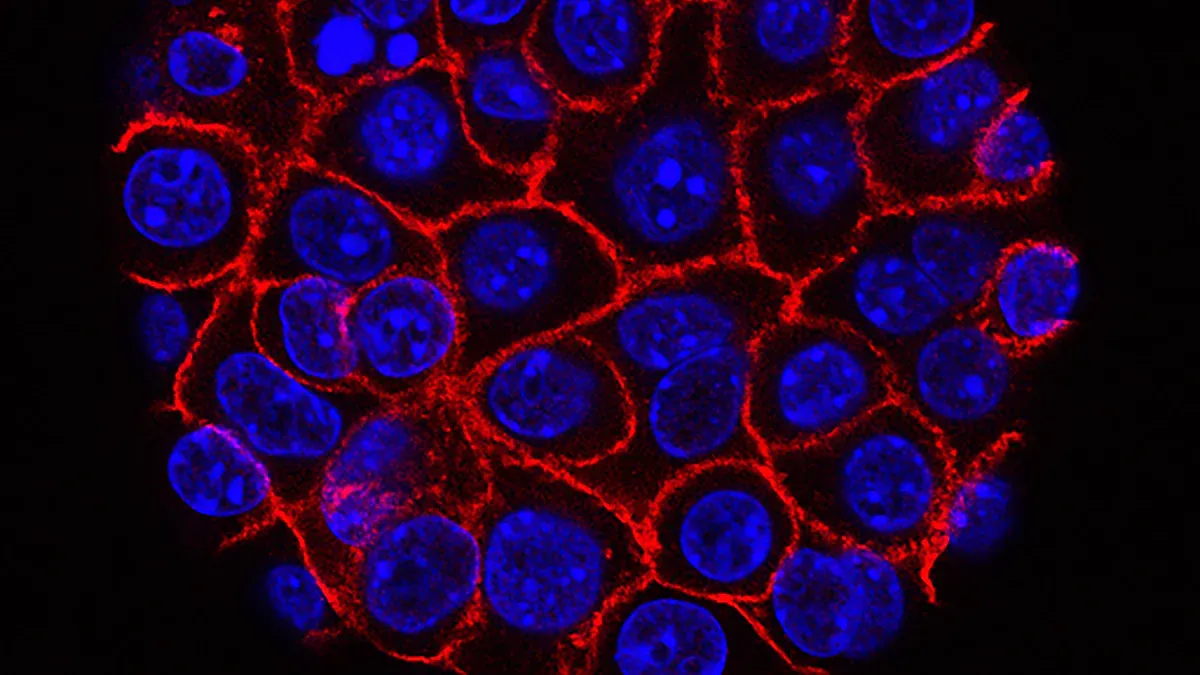
What Is Adhesion?
Cell adhesion describes how cells stick to each other and to their surroundings. This process helps tissues stay together and keeps cells in the right place. In the body, special molecules on the cell surface control adhesion. These molecules act like glue, holding cells together or attaching them to the extracellular matrix, which supports tissues.
Scientists have identified several key molecules that control cell adhesion. The table below shows some important types and their roles in cancer:
Molecular Component | Mechanism/Role in Cancer Adhesion | Functional Consequence |
|---|---|---|
Control cell-cell adhesion; changes in these molecules help cancer cells move | Promote cell migration and metastasis | |
L1CAM | Alters adhesion and signals cells to move | Increases cancer spread |
Integrins | Help cells attach to the matrix | Support invasion and movement |
CD44 | Connects with growth factor receptors | Affects how cells stick and migrate |
These molecules work together to keep normal cells in place. When their function changes, cells can break away and move to new locations.
Role in Tumors
In tumors, cell adhesion changes in important ways. Cancer cells often lose E-cadherin, which normally keeps them tightly connected. At the same time, they may gain N-cadherin, which helps them move. This process, called the "cadherin switch," allows cancer cells to become more mobile and invade other tissues.
Cancer cells with weak adhesion can escape from the main tumor.
Changes in integrins and cadherins help cancer cells survive in the bloodstream.
Some adhesion molecules, like N-cadherin, activate signals that make tumors more aggressive.
Tumor cells with altered adhesion can avoid the immune system and grow faster.
Over the past decade, researchers have learned that cell adhesion molecules do more than just hold cells together. They also help define cancer subtypes and guide treatment choices. For example, the loss of E-cadherin marks certain breast cancers, such as invasive lobular carcinoma. This knowledge helps doctors understand how tumors behave and how best to treat them.
Cell adhesion now stands as a key factor in cancer biology. By studying these molecules, scientists hope to find better ways to predict and stop cancer spread.
Microfluidic Device for Cancer Cell Adhesion
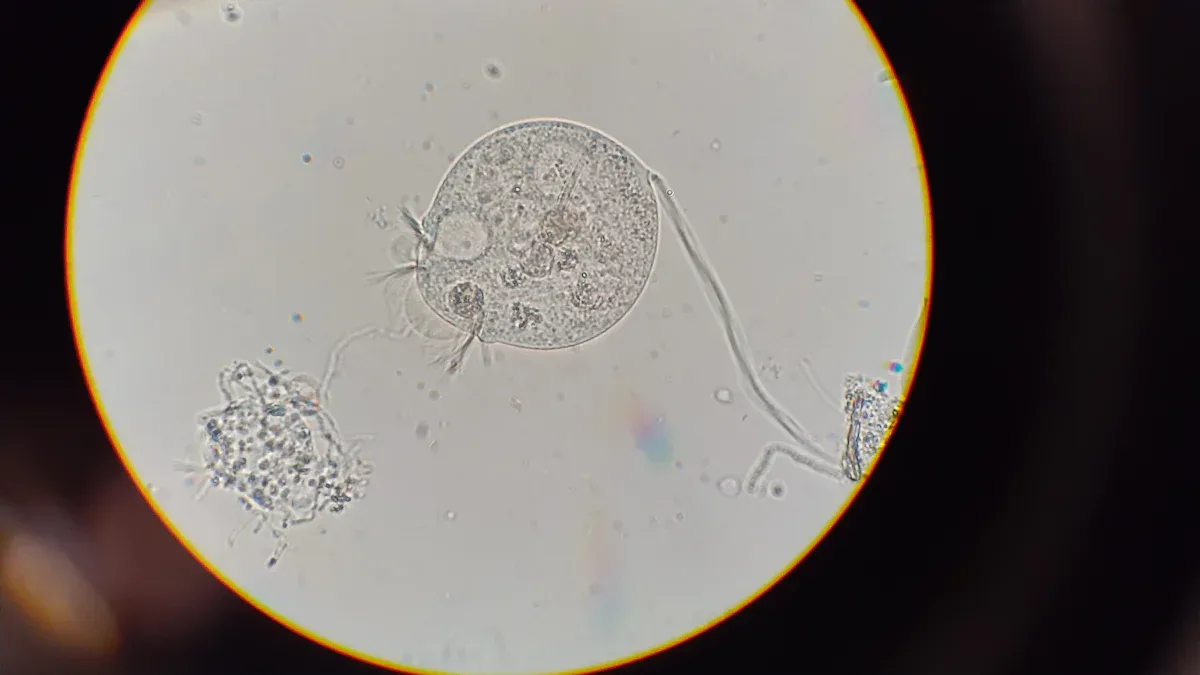
Device Design
Scientists have created a microfluidic device for cancer cell adhesion that sorts tumor cells based on how sticky they are. This device uses tiny chambers and channels to separate cells by their ability to stick to certain surfaces. The chambers are coated with proteins like fibronectin or special antibodies that attract cancer cells. When a sample flows through the device, cells with different stickiness levels behave in unique ways.
The microfluidic device for cancer cell adhesion includes several important features. It uses rows of small triangular posts, called a deterministic lateral displacement microarray, to separate cells by size. The tilt angle of these posts changes along the device, which helps sort cells more accurately. Another part, the trapping barrier microarray, has gaps that get smaller, mimicking the tight spaces found in blood vessels. This design allows the device to trap and test how cells squeeze through narrow spaces.
The table below shows some key features of the microfluidic device for cancer cell adhesion:
Feature | Description |
|---|---|
Deterministic Lateral Displacement Microarray | Tilted rows of triangular posts separate cells by size, with angles from 2.9° to 14.7°. |
Trapping Barrier Microarray | Rectangular array with gaps from 15 μm to 4 μm, mimicking capillary constrictions. |
Cell Transportability Measurement | Measures how cells squeeze through tight spaces, showing their stiffness and stickiness. |
High-throughput and Label-free | Sorts many cells quickly without special labels. |
Micro-filter Channel | Prevents cell clumping before sorting. |
Triangular Microposts | Posts with 30 μm sides and 27 μm height, arranged for best sorting. |
The materials used in the microfluidic device for cancer cell adhesion are also important. The base is made from a soft material called PDMS, shaped using special molds. The device has ridges and posts designed to guide cells along certain paths. The surfaces are treated to prevent cells from sticking in the wrong places. Scientists also coat the posts with antibodies that grab onto cancer cells, making the sorting process more accurate.
Note: The careful design of the microfluidic device for cancer cell adhesion helps scientists study how cancer cells behave in conditions similar to the human body.
How It Measures Stickiness
The microfluidic device for cancer cell adhesion does more than just sort cells by size. It also measures how well cancer cells stick to surfaces under flowing liquid, which mimics the forces found in blood vessels. The device traps single cells and exposes them to controlled fluid shear stress. This stress is like the force blood puts on cells as it moves through the body.
Scientists can change the amount and length of shear stress in the device. They use computer models to make sure the stress is even and the cells are trapped correctly. By watching how cells react—whether they stay stuck or get washed away—researchers can tell how sticky each cell is. Cells that hold on tightly may be less likely to spread, while those that let go easily might travel to other parts of the body.
The table below explains how the device measures cell responses to fluid shear stress:
Aspect | Description |
|---|---|
Cell trapping | Holds single cancer cells to test their stickiness. |
Shear stress control | Applies exact amounts of force to cells, using computer models for accuracy. |
Cellular response analysis | Watches how cells change shape or move when stressed. |
Analysis methods | Uses staining and other tests to see changes in cell behavior. |
Cellular responses | Looks for signs like increased growth or movement after stress. |
The microfluidic device for cancer cell adhesion can also mimic the tumor microenvironment. It creates 3D spaces filled with cancer cells, fibroblasts, and other tissue types. These setups let scientists see how cancer cells interact with their surroundings, including the extracellular matrix and immune cells. By adding flow and pressure, the device copies the real-life conditions that cancer cells face in the body.
Researchers have found that cancer cells respond differently to flow and pressure than they do in still conditions. For example, some cancer cells become more mobile and align with the direction of flow. Others show changes in proteins that control growth and movement. These findings help scientists understand which cells are more likely to spread and how treatments might stop them.
Tip: Microfluidic devices allow real-time imaging and testing of cancer cells in ways that traditional lab methods cannot. They help reveal the hidden behaviors of cancer cells under stress.
Scientists continue to improve the microfluidic device for cancer cell adhesion. They work to make the devices more sensitive, faster, and better at copying the real tumor environment. These advances could lead to better tests for cancer risk and new ways to personalize treatment.
Research Findings
Mouse Studies
Researchers used the microfluidic device to study how cancer cells behave in conditions similar to the human body. The device allowed them to control flow rates and add signals like TNF-α, which can cause inflammation. They found that cancer cell stickiness depends on both the force of the flowing liquid and the types of adhesion molecules on the cell surface. Integrins and selectins played important roles. When the device increased the flow, selectin binding became stronger, helping cells stick more firmly. This process mimicked the first steps of cancer cells moving through blood vessel walls.
Scientists tested different breast cancer cell lines, such as MCF7 and MDA-MB-231, in both the device and in mice. The results matched well. For example:
MCF7 cells caused lung metastasis in 80% of mice, while MDA-MB-231 and ACC-M cells did so in 100% of cases.
The device predicted these outcomes quickly, in just two days, compared to over three weeks for mouse experiments.
Researchers also tested a drug called AMD3100. The device showed that higher doses of the drug reduced cancer cell stickiness, which matched fewer lung metastases in mice.
These findings show that the microfluidic device can help study how cancer spreads and test new treatments.
Human Tissue Results
Tissue Sample Type | Adhesion Strength Observed | Notes on Variability or Interpretation |
|---|---|---|
Normal Breast Tissue | Strongly adherent cells | Consistently strong adhesion |
DCIS Tumors | Intermediate adhesion | Significant variability among patients |
Aggressive Breast Cancer | Weakly adherent cells | Includes invasive ductal and lobular carcinomas |
The table above shows how different human breast tissue samples performed in the microfluidic device. Normal breast cells stuck tightly to the surface. Aggressive cancer cells, including invasive types, showed weak adhesion. DCIS tumor cells had intermediate stickiness, but their results varied from patient to patient.
DCIS Insights
Studies of ductal carcinoma in situ (DCIS) reveal that changes in genes controlling cell adhesion and the cell skeleton increase the risk of cancer returning. Mutations in genes like MYO7A, SH2B2, and PDZD8 disrupt how cells hold together. Changes in cadherin genes also weaken tissue structure. These genetic changes make it easier for DCIS to progress or come back after treatment. Some types of DCIS, such as comedo lesions, have the highest risk of returning. Researchers found that keeping cell adhesion strong helps prevent DCIS from becoming invasive cancer. These findings suggest that measuring cell stickiness and looking for certain gene changes could help doctors predict which DCIS cases need more treatment.
Clinical Impact
Risk Assessment
Doctors need better ways to predict which breast cancer patients face a higher risk of their cancer spreading. Traditional methods, such as looking at tumor size or grade, do not always give clear answers. Measuring how cancer cells stick to their surroundings can help fill this gap. Scientists use biosensors in organ-on-a-chip models to watch how live cancer cells interact with their environment. These tools show how factors like nutrition or breast density affect cancer risk. By studying cell adhesion, researchers can see which tumors have a higher chance of spreading.
Gene studies also help doctors understand cancer risk. Researchers have found that certain genes control how sticky cancer cells are. They use this information to build risk models. These models predict how a patient’s cancer might behave and how the immune system will respond. Doctors can use these models to decide which patients need more careful monitoring or stronger treatment.
The microfluidic device for cancer cell adhesion gives doctors a new way to measure these properties in real time. This device sorts and tests cancer cells, showing which ones are more likely to break away and spread. With this information, doctors can make better decisions about each patient’s risk.
Note: Improved risk assessment helps doctors catch aggressive cancers early and avoid missing high-risk cases.
Reducing Overtreatment
Many patients with ductal carcinoma in situ (DCIS) receive surgery, radiation, or medicine even when their cancer may never spread. Current tests cannot always tell which DCIS cases are dangerous. The new device measures how strongly tumor cells stick to their surroundings. Weakly adherent cells have a higher chance of becoming invasive. By finding these cells, doctors can spot which patients need more treatment and which do not.
This approach helps avoid unnecessary procedures for low-risk patients. Doctors can use the results to recommend less aggressive care when it is safe. Patients with low-risk DCIS may skip surgery or radiation, reducing side effects and stress. At the same time, those with high-risk cells can get the care they need right away.
Current DCIS decisions rely on size and grade, which do not always predict risk.
The device identifies weakly adherent cells, showing higher invasive potential.
Doctors can match treatment to the real risk, not just the appearance of the tumor.
Experts stress the importance of avoiding overtreatment. Patients should not face aggressive surgery or medicine unless it is truly needed.
Personalizing Treatment
Every breast cancer patient is different. Some tumors respond well to certain drugs, while others do not. Measuring cell adhesion helps doctors create treatment plans that fit each patient. Prognostic models based on cell adhesion genes show how likely a tumor is to spread. These models also reveal how the immune system and tumor environment affect outcomes.
Doctors can use information from the microfluidic device for cancer cell adhesion to guide therapy choices. For example, they can test how a patient’s cancer cells react to different treatments. If a tumor has weak adhesion, doctors may choose stronger therapies or closer follow-up. If the cells stick tightly, less aggressive care may be enough.
Circulating tumor cells (CTCs) can be found in blood using adhesion markers. This allows doctors to monitor cancer without surgery.
CTCs show how the tumor changes over time, helping doctors adjust treatment as needed.
Adhesion measurements can predict which patients will respond to immunotherapy or targeted drugs.
Personalized treatment means better results and fewer side effects. Patients get the care that matches their unique cancer, not a one-size-fits-all plan.
Note: Before these new tools become standard, researchers must test them in larger and more diverse groups. They need to make sure the results work for everyone, not just a small group of patients. Doctors also need clear guidelines on how to use the results in real life.
Future Directions
Larger Studies
Researchers plan to expand their studies on cancer cell adhesion and metastasis prediction. They want to make sure the findings apply to more people and different cancer types. Scientists suggest several next steps:
Measure how cancer cell stickiness changes over time. This helps capture how cells form and break their attachments.
Test adhesion strength on different surfaces, such as cadherin, collagen, or laminin. These materials reflect the variety found in the body.
Use many types of cancer cell lines. This broadens the results and makes them more useful.
Try the bead-pipette assay as a screening tool in clinics. Doctors could use it to see if new drugs help stop cancer from spreading.
Researchers also want to improve liquid biopsy techniques. These tests look for cancer cells or DNA in blood. Better tests could help doctors find cancer spread earlier and monitor patients more closely.
Other Cancers
The microfluidic device that measures cell stickiness can work for cancers beyond breast cancer. Scientists can change the antibodies on the device to capture different cancer cells. For example, they have used anti-EpCAM antibodies to catch prostate cancer cells. By switching the capture chemistry, the device can target circulating tumor cells from many cancer types. This flexibility means doctors could use the same technology to study and predict metastasis in lung, colon, or other cancers.
Expert Views
Many oncology experts see great promise in measuring cancer cell stickiness. They believe this approach can help predict which tumors will spread and guide treatment choices. Experts highlight the need to understand how cancer cells interact with their surroundings. They point out that new therapies could target adhesion molecules, like integrins or focal adhesion kinase, to stop cancer from moving. Some drugs already show a strong reduction in metastasis by blocking these pathways.
Experts also note challenges. Tumor cells can behave differently, and the tumor environment is complex. Advanced lab models and personalized medicine will help address these issues. New diagnostic protocols may soon include markers for cell adhesion and tumor microenvironment changes, helping doctors spot aggressive cancers earlier.
Measuring cancer cell stickiness offers a new way to predict breast cancer spread and personalize treatment. Recent studies show that adhesion strength can accurately identify tumors with high metastatic risk. Immune-related proteins and cell adhesion molecules play key roles in risk prediction. Microfluidic devices improve accuracy, speed, and cost compared to older methods. They also detect drug resistance genes for better care.
Ongoing research and larger studies will help doctors use these tools to guide treatment. Staying informed about new cancer diagnostics can help patients and families make better decisions.
FAQ
What does "cancer cell stickiness" mean?
Cancer cell stickiness describes how tightly cancer cells attach to each other or to their surroundings. Scientists call this property "cell adhesion." Cells with weak adhesion can break away and move more easily.
How does the microfluidic device work?
The microfluidic device sorts cancer cells by their stickiness. It uses tiny channels and special coatings to test how well cells hold on when liquid flows over them. Less sticky cells let go faster.
Why is measuring cell adhesion important for breast cancer?
Doctors use cell adhesion to predict if cancer will spread. Weakly adhesive cells often travel to other parts of the body. Measuring stickiness helps doctors find high-risk tumors and choose the best treatment.
Can this device help avoid unnecessary treatment?
Yes! The device can show which tumors are less likely to spread. Doctors may recommend less aggressive care for patients with sticky, low-risk cancer cells.
Will this technology work for other cancers?
Researchers believe the device can test many cancer types. By changing the surface coatings, scientists can study stickiness in lung, colon, or prostate cancer cells.
See Also
Different Cancer Types Commonly Associated With AIDS Diagnosis
Recognizing Symptoms And Understanding Invasive Lobular Carcinoma
A Guide To Large Cell Lung Cancer With Rhabdoid Features
Key Molecular Characteristics Of Lung Giant Cell Carcinoma
Identifying Symptoms And Facts About Inflammatory Breast Cancer

