Understanding CAR-T Cell Therapy and Its Role in Cancer Treatment
Imagine if your immune system could be trained to fight cancer as effectively as it fights infections. CAR-T cell therapy makes this possible. This innovative treatment uses your own T-cells, which are a type of white blood cell, to recognize and destroy cancer cells. Scientists reprogram these cells in a lab to target specific proteins found on cancer cells. Once infused back into your body, these modified cells become powerful cancer fighters. The Rise of CAR-T Cell Therapy: Revolutionizing Blood Cancer Treatment highlights how this approach is transforming lives, especially for patients with leukemia and lymphoma.
Key Takeaways
CAR-T cell therapy uses your own immune cells to fight cancer.
These cells are changed to attack cancer cells directly.
This treatment works well for blood cancers, with success rates up to 81%.
Some patients may have side effects like fever or low blood pressure.
Doctors watch closely and treat these side effects if they happen.
Scientists are studying how to use CAR-T for other cancers and diseases.
Joining clinical trials can help patients try new treatments early.
How CAR-T Cell Therapy Works
Collecting and isolating T-cells
The first step in CAR-T cell therapy begins with collecting your T-cells, which are a type of immune cell. Doctors use a process called leukapheresis to separate these cells from your blood. During this procedure, your blood is drawn, and a machine isolates the T-cells before returning the rest of your blood to your body. This ensures that only the cells needed for therapy are collected.
Once isolated, these T-cells undergo careful preparation in a laboratory. Researchers activate them to ensure they are ready for genetic modification. Proper activation is critical because it helps the cells respond effectively to the changes introduced during the next phase.
Evidence Description | Source |
|---|---|
T cells are isolated from patients' peripheral blood and modified ex-vivo using gene transfer through viral vectors, which integrate the CAR cassette into the T cell’s DNA. | |
Proper activation of T cells is critical for efficient CAR gene transduction and proliferation of transduced T cells. |
Genetic modification to create CAR-T cells
After collecting your T-cells, scientists reprogram them to become cancer-fighting cells. This process involves introducing a special receptor called a chimeric antigen receptor (CAR) into the T-cells. The CAR enables your cells to recognize and attack specific proteins found on cancer cells.
The genetic modification process uses advanced techniques, such as viral vectors, to insert the CAR gene into the DNA of your T-cells. These vectors act like delivery vehicles, ensuring the CAR gene integrates seamlessly. Researchers also enhance the functionality of your modified cells by incorporating costimulatory molecules. These molecules boost the activation and persistence of your CAR-T cells, making them more effective in targeting tumors.
Mechanism | Description |
|---|---|
Genetic Modification | T-cells are genetically modified to express chimeric antigen receptors (CARs) that allow them to recognize specific tumor cells. |
Costimulatory Molecules | Incorporation of costimulatory molecules enhances T-cell activation and function against tumors. |
T-cell Persistence | Strategies such as using central memory T-cells and introducing cytokine genes (e.g., IL-12, IL-15) improve the persistence and antitumor activity of CAR T-cells. |
Overcoming Immunosuppression | CAR T-cells can be engineered to counteract immunosuppressive factors in the tumor microenvironment, such as TGFβ and CTLA-4. |
This genetic engineering step is crucial for ensuring your T-cells can survive and thrive in the challenging environment of your body, especially when facing cancer's defenses.
Infusion of CAR-T cells into the patient
Once your T-cells are modified and expanded in the lab, they are ready to be infused back into your body. Before the infusion, you may undergo a short course of chemotherapy to prepare your immune system. This step creates a favorable environment for the CAR-T cells to function effectively.
The infusion process is straightforward and resembles a blood transfusion. Your CAR-T cells are delivered directly into your bloodstream, where they begin their mission to locate and destroy cancer cells. These cells work tirelessly, targeting cancer with precision and adapting to challenges within your body.
💡 Tip: After infusion, your healthcare team closely monitors you for potential side effects, such as cytokine release syndrome (CRS). CRS can cause symptoms like fever, fatigue, and low blood pressure. Early detection and management ensure your safety during this critical phase.
A new endpoint called CAR T EFS (Intention-to-Treat Event-Free Survival) has been proposed to measure the effectiveness of this therapy. It tracks outcomes from the start of the CAR-T treatment process, offering a comprehensive view of its benefits.
Clinical Evidence Challenges in CAR-T Cell Therapy |
|---|
Limited sample size in clinical studies |
Use of surrogate endpoints |
Immaturity of evidence and extrapolation of long-term endpoints |
Safety issues that may emerge over time |
Your journey through CAR-T cell therapy involves cutting-edge science and personalized care, giving you a powerful tool to fight cancer.
The Rise of CAR-T Cell Therapy: Revolutionizing Blood Cancer Treatment
Approved treatments for leukemia and lymphoma
CAR-T cell therapy has transformed the treatment landscape for blood cancers like leukemia and lymphoma. You may find it remarkable that this therapy targets specific proteins on cancer cells, such as CD19 and BCMA, leading to impressive outcomes. For example:
Patients with aggressive B-cell lymphomas have shown complete response (CR) rates of 40–54%.
Mantle cell lymphoma patients have achieved CR rates of 67%.
Indolent B-cell lymphoma patients have experienced CR rates as high as 74%.
In relapsed B-cell acute lymphoblastic leukemia (B-ALL), CR rates range from 71–81%.
These results highlight the effectiveness of CAR-T therapy in cases where traditional treatments have failed. The FDA has approved several CAR-T therapies, offering new hope to patients with relapsed or refractory blood cancers. Clinical trials have also demonstrated a median response rate of 66.4% in patients with B-cell non-Hodgkin lymphoma (B-NHL). This success underscores the growing adoption of CAR-T therapy as a standard treatment option.
Year | Estimated Eligibility for CAR-T Therapies |
|---|---|
2017 | 2.7% (95% CI, 2.6%-2.7%) |
2023 |
These statistics reflect the increasing accessibility of CAR-T therapy, making it a revolutionary option for blood cancer treatment.
Expanding research into other cancers
While CAR-T therapy has excelled in treating blood cancers, researchers are now exploring its potential in other cancers. You might be intrigued to learn about its application in solid tumors, which present unique challenges due to their complex microenvironments. For instance:
CD47-targeting immune checkpoint inhibitors are in clinical trials for both solid tumors and blood cancers, with one therapy already in Phase III.
GD2-CAR-T therapy has shown promise in reducing tumor volume in patients with diffuse midline gliomas, although durability remains a challenge.
Cytokine-enhanced CAR-T therapies, such as huCART19-IL18, are being developed to improve responses in solid tumors.
Additionally, CAR-T therapy is being tested in autoimmune diseases. A recent study showed that 14 out of 15 patients with refractory systemic lupus erythematosus achieved drug-free remission after receiving CD19-CAR-T therapy. These advancements demonstrate the versatility of CAR-T technology and its potential to address a broader range of diseases.
As research continues, you can expect CAR-T therapy to expand its reach, offering hope to patients with conditions previously considered untreatable.
Effectiveness and Safety
Success rates and remission statistics
CAR-T cell therapy has demonstrated remarkable success in treating certain cancers, particularly blood cancers. You might find it encouraging to know that clinical studies report high remission rates. For example:
Statistic | Value |
|---|---|
Complete initial remission rate | 93% (40 of 43) |
MRD− CR rate in all subjects | 89% (40 of 45) |
MRD− CR rate among CAR T infusion | 93% (40 of 43) |
1-year remission retention | ~50% |
Molecular CR by day 63 | 65% (17 of 27) |
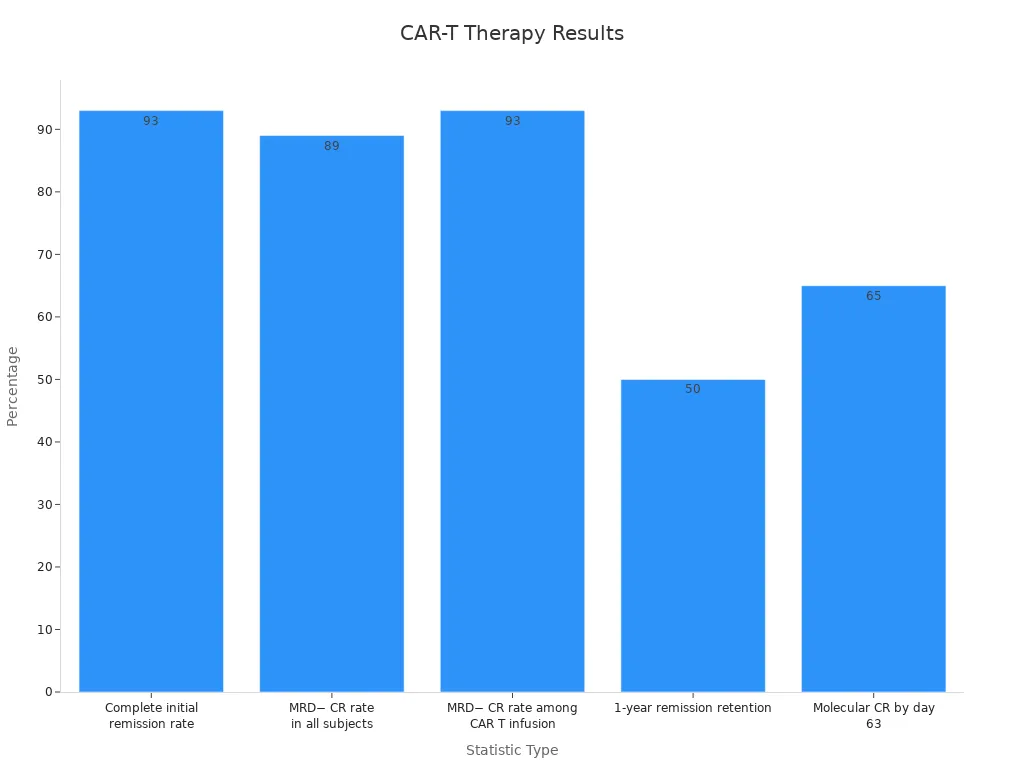
These statistics highlight the therapy's potential to achieve complete remission in many patients. However, the duration of remission can vary. Factors such as the disease's status at the time of infusion and the use of combined treatments can influence outcomes. For central nervous system lymphoma (CNSL), studies show that CAR-T therapy provides sustained efficacy, although relapse risks remain a concern.
Beyond clinical outcomes, CAR-T therapy also improves your quality of life. A meta-analysis revealed significant improvements in six areas, including general health, pain, fatigue, and social function. These benefits often become noticeable within three months of treatment, offering you not just hope for survival but also a better quality of life.
Managing side effects like cytokine release syndrome
While CAR-T cell therapy is effective, it can cause side effects. One of the most common is cytokine release syndrome (CRS). This condition occurs when your immune system reacts strongly to the therapy, leading to inflammation. Symptoms often include fever, fatigue, and low blood pressure. These can appear within hours or days after treatment, but in some cases, symptoms may take up to two weeks to develop.
The American Society for Transplantation and Cellular Therapy (ASTCT) has developed guidelines to grade CRS severity. These guidelines consider factors like temperature, blood pressure, and oxygen levels. Mild cases may only require monitoring, while severe cases might need medications like tocilizumab or corticosteroids to control inflammation.
Adverse Effect | LBCL | FL | ALL | MM |
|---|---|---|---|---|
CRS (Any grade) | 78% | 49-78% | 82% | 76% |
Neurotoxicity (Any grade) | 41% | 37-56% | 29% | 10.5% |
CRS (Grade ≥3) | 6-18% | 0-6% | 26% | 11% |
Neurotoxicity (Grade ≥3) | 16-19% | 3-15% | 12% | 8% |
Neurotoxicity is another potential side effect, though it occurs less frequently. Symptoms can include confusion, headaches, or seizures. Your healthcare team will monitor you closely to manage these risks effectively. Supportive care, routine lab tests, and specific interventions ensure that side effects are addressed promptly, keeping you safe throughout the treatment process.
Safety protocols during and after treatment
Safety is a top priority in CAR-T cell therapy. Before treatment, your healthcare team conducts thorough evaluations to ensure you are a suitable candidate. During the therapy, they follow established guidelines, such as those from the National Comprehensive Cancer Network (NCCN) and ASTCT, to monitor your response and manage any complications.
After the infusion, you will stay under close observation for several weeks. This period allows your team to detect and treat any side effects early. Routine lab tests help track your progress, while multivariate analyses identify risk factors for severe events like CRS or neurotoxicity.
Long-term safety protocols focus on your overall survival (OS) and event-free survival (EFS). These measures help assess the therapy's effectiveness over time. By adhering to these protocols, your healthcare team ensures that you receive the best possible care, minimizing risks while maximizing the benefits of CAR-T therapy.
The Rise of CAR-T Cell Therapy: Revolutionizing Blood Cancer Treatment has not only improved survival rates but also set new standards for patient safety. With ongoing advancements, this therapy continues to offer hope to patients facing challenging diagnoses.
Patient Experience
Steps to prepare for CAR-T therapy
Preparing for CAR-T therapy involves several important steps to ensure the best possible outcome. Your healthcare team will first assess your eligibility through a detailed screening process. This evaluation includes blood tests, imaging scans, and a review of your medical history. These steps help determine if CAR-T therapy is the right option for you.
To collect your T-cells, doctors use a procedure called leukapheresis. This process requires careful planning to achieve a sufficient T-cell yield while managing vascular access. Specialists also work to eliminate contaminating cell types, ensuring the collected cells are optimal for modification.
💡 Tip: Staying hydrated and following your doctor’s instructions before leukapheresis can improve the quality of your T-cell collection.
What happens during the treatment process
CAR-T therapy follows a structured process that spans several stages. Initially, you undergo screening to confirm your eligibility. Once your T-cells are collected and genetically modified, they are prepared for infusion.
Component | Steps | Time Estimate (hours) | Description |
|---|---|---|---|
Screening | 1-4 | 30.5 - 52.4 | Initial patient evaluation and eligibility assessment. |
CAR T-cell Preparation | 5-9 | 71.5 - 192.8 | Preparation and administration of CAR-T cells, including infusion and monitoring. |
Acute Management | 10 | 156.7 - 424.8 | Management of acute side effects post-infusion, requiring close monitoring. |
Long-term Management | 11-13 | 4.0 - 13.1 | Ongoing care and monitoring of patients after initial treatment. |
During the infusion, your healthcare team monitors you closely for any immediate side effects. This phase requires precision and constant observation to ensure your safety.
Recovery and follow-up care
Recovery after CAR-T therapy varies for each patient. You may need to stay in the hospital for a few weeks to monitor for side effects like cytokine release syndrome (CRS) or neurotoxicity. These conditions, while manageable, require prompt attention.
Long-term follow-up care focuses on your overall health and quality of life. Regular check-ups and lab tests help track your progress. Many patients report meaningful changes, such as symptom improvement and a return to normalcy.
Theme | Description |
|---|---|
Meaningful changes | Patients consider meaningful change as symptom improvement and return to normalcy, with evaluations varying based on their understanding of the disease. |
Treatment-related distress | Patients reported intensified distress due to concerns about treatment efficacy and adverse reactions, experiencing a range of emotions such as fear, anger, and frustration. |
🩺 Note: Open communication with your healthcare team during recovery ensures that any concerns are addressed promptly, helping you achieve the best possible outcome.
Eligibility and Access
Criteria for qualifying for CAR-T therapy
CAR-T therapy is not suitable for everyone. Doctors use specific criteria to determine if you qualify for this treatment. These criteria ensure that the therapy is both safe and effective for you.
Eligibility often depends on the type of cancer you have. CAR-T therapy is currently approved for certain blood cancers, such as B-cell lymphoma and acute lymphoblastic leukemia.
You need to meet minimum health requirements. These include having good organ function and a stable performance status, which means you can carry out daily activities without significant limitations.
Doctors also evaluate your medical history. Conditions like graft-versus-host disease (GVHD), recent infections, or central nervous system (CNS) issues may affect your eligibility due to potential risks like neurotoxicity.
Criteria Type | Details |
|---|---|
Based on FDA-approved indications and pivotal studies. | |
Considerations for Evaluation | GVHD, uncontrollable infections, recent donor-lymphocyte infusion (DLI), and CNS pathology. |
Protocol-Specific Criteria | Age, weight, prior treatments (e.g., HSCT, CD19-targeted therapy), and organ function. |
Interestingly, real-world studies show that even older or frail patients with multiple health issues can tolerate CAR-T therapy. However, there is no universal agreement on how to assess eligibility, which means your doctor will carefully weigh the risks and benefits before recommending this treatment.
💡 Tip: If you’re unsure about your eligibility, ask your healthcare provider for a detailed evaluation. They can guide you through the process and explain your options.
Accessing treatment at specialized centers
CAR-T therapy is only available at specialized centers. These facilities have the expertise and resources needed to administer this complex treatment safely.
Evidence Type | Description |
|---|---|
Many treatment centers are located in large academic hospitals, which may be far from your home. | |
Outpatient Feasibility | While outpatient therapy is possible, severe side effects often require hospitalization. |
Real-World Evidence | Patients treated in real-world settings have outcomes similar to those in clinical trials. |
Accessing these centers can be challenging if you live in a rural area or far from a major city. Travel and accommodation costs may also add to the burden. Despite these challenges, studies show that patients treated in real-world settings achieve results comparable to those in clinical trials. This suggests that CAR-T therapy can benefit a wide range of patients when administered by experienced teams.
🏥 Note: If you’re considering CAR-T therapy, ask your doctor about the nearest specialized center. Some hospitals offer support programs to help with travel and lodging.
Future of CAR-T Therapy
Advances in CAR-T technology
CAR-T therapy continues to evolve, offering new possibilities for cancer treatment. Researchers have identified mechanisms that cause resistance in CAR-T cells, which could lead to therapies with longer-lasting effects. You might find it fascinating that scientists are exploring allogeneic CAR-T therapies, which use cells from healthy donors. These therapies aim to reduce rejection risks and make treatment more accessible.
Another exciting development involves using CAR-T therapy earlier in treatment plans for blood cancers. This approach could improve outcomes by targeting cancer cells before they become resistant to other treatments. Advances in gene editing and antibody incorporation are also helping overcome challenges like therapy failures.
🧬 Note: These breakthroughs highlight the potential of CAR-T technology to become even more effective and widely available in the future.
Expanding applications to solid tumors
Solid tumors present unique challenges for CAR-T therapy, but researchers are making progress. You may be intrigued to learn that combining CAR-T cells with oncolytic viruses is being tested to treat solid tumors like liver cancer. This combination enhances the ability of CAR-T cells to penetrate the tumor’s microenvironment.
Experimental therapies are targeting proteins specific to solid tumors, such as GD2 in glioblastoma and glypican-3 in gliomas. These approaches involve innovative delivery methods, including intraventricular administration and direct injection into the tumor site.
Key advancements in solid tumor applications:
CD47-targeting immune checkpoint inhibitors are being studied for thyroid cancer.
Cytokine-enhanced CAR-T therapies, like huCART19-IL18, aim to improve responses in solid tumors.
Combining CAR-T cells with gene editing tools is helping overcome immunosuppressive barriers in solid tumors.
These efforts could expand CAR-T therapy beyond blood cancers, offering hope to patients with hard-to-treat conditions.
Ongoing clinical trials and research
Clinical trials are driving the future of CAR-T therapy. Nearly 1,000 trials have been registered in the past eight years, reflecting robust research activity. These studies focus on improving safety, effectiveness, and expanding applications. For example, trials are exploring new target proteins like EGFRvIII for neuroblastoma and glypican-3 for gliomas.
Target Proteins | Administration Methodology | |
|---|---|---|
Neuroblastoma | EGFRvIII | IV |
Glioblastoma | GD2 | Intraventricular |
Gliomas | Glypican-3 | Direct to tumor site |
Hepatocellular Carcinomas | N/A | Direct to tumor site |
The CAR-T cell therapy market is projected to grow from USD 4.6 billion in 2025 to USD 15.2 billion by 2035, driven by advancements in molecular research and personalized cancer treatments. However, challenges like high costs and the need for improved CAR constructs remain.
🌟 Tip: Stay informed about clinical trials and emerging therapies. These developments could shape the future of cancer treatment and offer new options for patients.
CAR-T cell therapy has redefined cancer treatment, offering you hope when other options seem limited. Its success in blood cancers like leukemia and lymphoma demonstrates its transformative potential. Long-term studies reveal sustained remission in some patients for over a decade. For example:
Study Type | Patient Group | Outcome | Duration of Remission |
|---|---|---|---|
Long-term follow-up | 2 adult patients | Sustained remission | |
Long-term follow-up | 43 patients with B-cell malignancies | 51% complete remission | ≥3 years |
Meta-analysis | 2,134 patients with RR/B-ALL | Favorable overall survival | N/A |
Retrospective analysis | 809 patients with R/R DLBCL | Greater efficacy with Axicabtagene-ciloleucel | N/A |
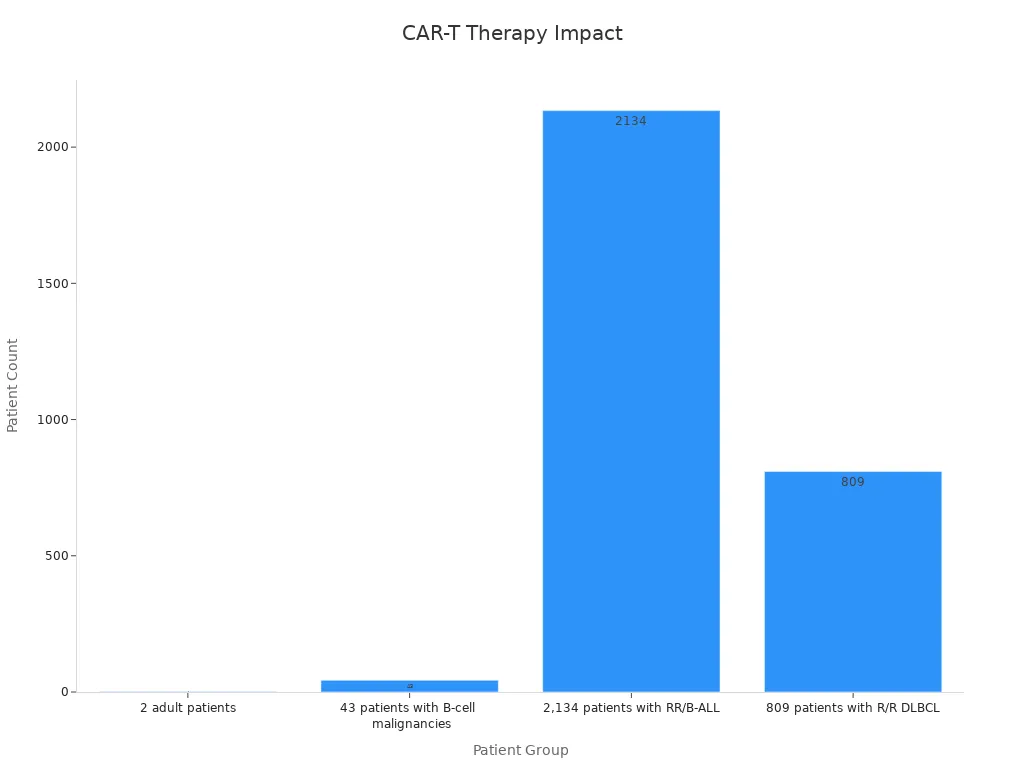
Ongoing research promises even greater advancements, expanding its reach to solid tumors and other diseases. This therapy represents a brighter future for cancer care.
FAQ
What types of cancer can CAR-T therapy treat?
CAR-T therapy is approved for certain blood cancers, including B-cell lymphoma and acute lymphoblastic leukemia. Researchers are also exploring its potential for solid tumors and autoimmune diseases.
How long does it take to complete CAR-T therapy?
The entire process, from T-cell collection to infusion, typically takes 2-3 weeks. Recovery and follow-up care may extend the timeline, depending on your response to treatment.
Are there risks involved with CAR-T therapy?
Yes, CAR-T therapy can cause side effects like cytokine release syndrome (CRS) and neurotoxicity. Your healthcare team monitors you closely to manage these risks effectively.
🩺 Tip: Early detection of side effects ensures better outcomes. Always report symptoms promptly.
Can older adults receive CAR-T therapy?
Yes, many older adults tolerate CAR-T therapy well. Doctors assess your overall health and medical history to determine if you qualify for treatment.
Is CAR-T therapy covered by insurance?
Most insurance plans cover CAR-T therapy for FDA-approved indications. However, coverage varies, so check with your provider to understand your benefits and out-of-pocket costs.
💡 Note: Some hospitals offer financial assistance programs to help with treatment costs.
See Also
Essential Insights About Carcinoid Tumors You Need
Key Information About Islet Cell Carcinoma Explained
Choriocarcinoma: Definition, Symptoms, And Treatment Options
Cholangiocarcinoma: Important Features And Understanding Its Impact

