Ultra-Rapid Genetic Testing Transforms Brain Tumor Surgery
Ultra-rapid genetic testing in brain tumor surgery now delivers mutation results within minutes, not weeks, giving surgeons real-time data to guide each step. Surgeons use this information to make decisions that directly affect patient safety and recovery.
Recent advances show impressive accuracy in tumor classification:
Metric
Value/Range
Description
Accuracy in tumor differentiation
Distinguishes brain tumors from metastatic tumors
F1 scores per tumor class
> 0.83 (mean ~0.96)
Strong performance across different tumor types
Patients benefit from faster answers and more precise surgery. Surgeons can detect key mutations, such as IDH1 R132H and BRAF V600E, in about 15 minutes. This speed allows them to adjust the operation immediately, improving both safety and outcomes.
Key Takeaways
Ultra-rapid genetic testing delivers mutation results within minutes during brain tumor surgery, helping surgeons make faster, safer decisions.
This testing detects key mutations like IDH1 R132H and BRAF V600E with high accuracy, even when tumor cells are few, improving tumor removal precision.
Combining genetic testing with imaging and other tools gives surgeons a clearer picture of tumor boundaries, reducing the chance of leaving cancer cells behind.
Clinical trials show ultra-rapid testing is reliable, cost-effective, and can provide detailed tumor classification during surgery, speeding up diagnosis from weeks to hours.
Future advances aim to automate and expand this technology to other cancers, promising faster diagnosis and better treatment for many patients.
Need for Real-Time Data
Tumor Margin Challenges
Surgeons face major obstacles when trying to identify the exact edges of brain tumors. Tumor cells, especially in aggressive types like glioblastoma, often invade healthy brain tissue. This makes it hard to see where the tumor ends and normal tissue begins. Even with the best surgical skills, visual cues alone are not enough.
Tumor cells can spread beyond what the eye can see.
Incomplete removal of tumor tissue increases the risk of recurrence.
Traditional frozen-section analysis takes time and samples only small areas, delaying decisions.
Surgeons often rely on frozen tissue analysis and expert pathologists during surgery. However, this process is slow and labor-intensive. The shortage of neuropathologists adds to the challenge. As a result, surgeons may leave behind microscopic tumor cells, which can worsen patient outcomes.
Emerging tools, such as AI-powered DNA decoding and rapid molecular diagnostics, now offer real-time information. These technologies help surgeons decide how much tissue to remove and whether to use tumor-killing drugs right away. By knowing the tumor’s molecular type during surgery, doctors can tailor their approach to each patient, improving the chances of a better recovery.
Imaging Limitations
Imaging techniques, such as intraoperative MRI and ultrasound, help surgeons see the tumor during surgery. However, these tools have important limitations:
Conventional imaging often fails to clearly show the true boundaries of a tumor.
Advanced MRI methods, like diffusion tensor imaging, struggle to map complex brain structures. Up to 90% of white matter areas contain crossing fibers, which current imaging cannot fully resolve.
Imaging cannot always tell the difference between tumor infiltration and swelling (edema).
These challenges mean that even the best imaging may miss small clusters of tumor cells. As a result, surgeons need better, faster ways to detect tumor margins. Real-time genetic testing fills this gap by providing molecular data that imaging alone cannot offer. This combination of tools can lead to more complete tumor removal and improved patient outcomes.
Ultra-Rapid Genetic Testing in Brain Tumor Surgery

How ddPCR Works
Ultra-rapid genetic testing in brain tumor surgery uses a special method called droplet digital PCR (ddPCR). This technology helps doctors find important genetic changes in tumor tissue during surgery. The process combines the fast cycling of Extreme PCR with the accuracy of standard ddPCR. Doctors use an ultra-rapid DNA extraction method that keeps the sample safe. The steps include bead homogenization, heat incubation, and centrifugation with gentle buffers. This careful process protects the DNA droplets and makes sure the test works well.
The entire workflow, from tissue to result, takes about 15 minutes.
Doctors can repeat the test during surgery to check for tumor cells in real time.
The test targets hotspot mutations, such as IDH1 R132H and BRAF V600E, which are common in aggressive brain tumors.
Ultra-rapid genetic testing in brain tumor surgery gives surgeons the power to make quick decisions. They can see if the tumor has certain mutations and adjust their surgical plan right away. This method works well in the operating room and fits with other tools surgeons use. Unlike other molecular tests, this approach offers both speed and accuracy, making it unique for use during surgery.
Note: UR-ddPCR stands out because it combines unmatched speed with the ability to measure tumor cells accurately. Other methods, like methylation profiling or next-generation sequencing, take much longer and cannot provide results during surgery.
Speed and Sensitivity
Speed matters when surgeons need answers fast. Ultra-rapid genetic testing in brain tumor surgery delivers results much faster than traditional genetic tests. Standard methods, such as HRM/sequencing and next-generation sequencing (NGS), can take days. Even other rapid tests, like the Idylla test or immunohistochemistry (IHC), need over an hour. In contrast, UR-ddPCR can give results in about 15 minutes, making it ideal for use during surgery.
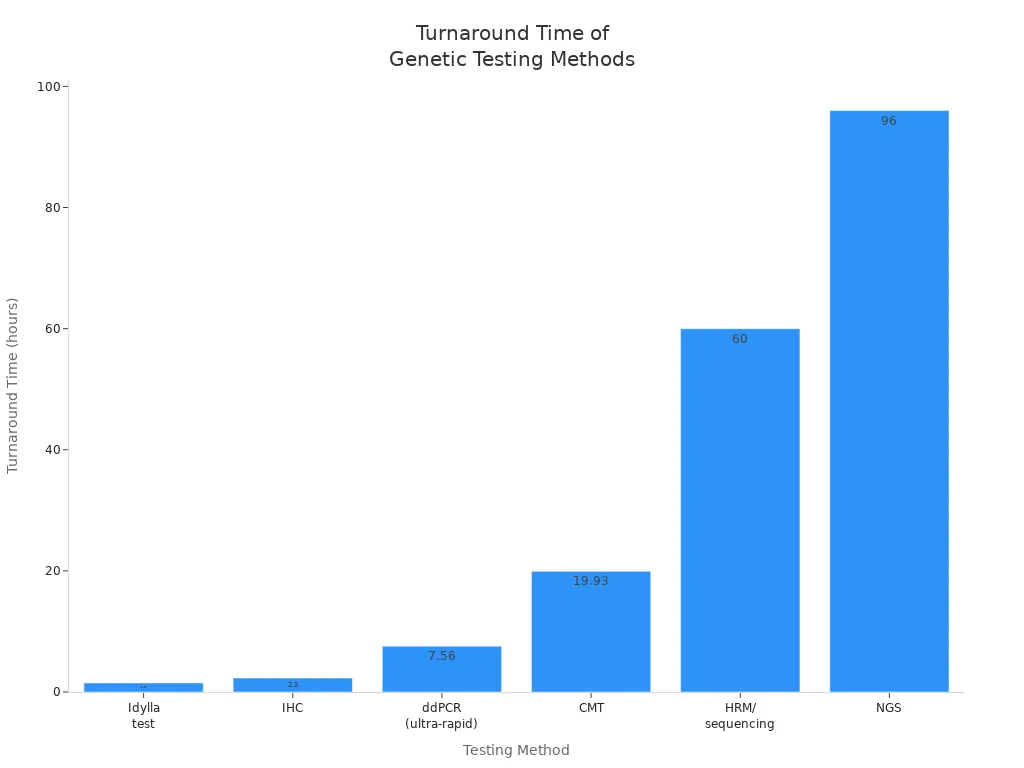
Testing Method | Turnaround Time (TAT) | Notes |
|---|---|---|
ddPCR (ultra-rapid) | ~7.56 hours | Significantly faster than CMT |
Clinical Microbiological Testing (CMT) | ~19.93 hours | Traditional microbiological culture-based |
Idylla test (rapid BRAF test) | 92 minutes (~1.5 hours) | Faster than ddPCR in specific rapid tests |
Immunohistochemistry (IHC) | 140 minutes (~2.3 hours) | Faster than ddPCR in specific rapid tests |
HRM/sequencing | 2–3 days | Traditional genetic testing, slower than ddPCR |
Next-Generation Sequencing (NGS) | 3–5 days | Traditional genetic testing, slower than ddPCR |
UR-ddPCR does more than work quickly. It also shows high sensitivity and specificity, which means it can find even small amounts of tumor DNA and rarely gives false results. In clinical studies, UR-ddPCR matched the results of standard ddPCR, which is known for its accuracy. For example, in a study with 49 samples from 13 glioma cases, UR-ddPCR showed full agreement with the gold-standard method. This reliability gives surgeons confidence to use the results for important decisions.
The test detects key mutations, such as IDH1 R132H and BRAF V600E. These mutations help doctors know what type of tumor they are dealing with and how aggressive it might be. By finding these mutations during surgery, doctors can remove more of the tumor while protecting healthy brain tissue. This approach lowers the risk of leaving tumor cells behind and improves patient outcomes. The technology was tested on 75 samples from 22 brain tumor surgeries and showed results that matched standard genetic sequencing.
Ultra-rapid genetic testing in brain tumor surgery allows doctors to check for tumor cells multiple times during one operation. This repeated testing helps them find any remaining tumor cells before finishing the surgery. As a result, patients have a better chance of recovery and a lower risk of the tumor coming back.
Validation and Accuracy
Clinical Testing
Ultra-rapid genetic testing has undergone rigorous clinical evaluation in real-world brain tumor surgeries. A recent clinical trial at the University of Nottingham and Nottingham University Hospitals NHS Trust tested this technology during 50 brain tumor operations. The team used nanopore-based sequencing with intraoperative methylome classification. This method delivered diagnostic results in under two hours, a dramatic improvement over the traditional six to eight-week timeline. Surgeons and pathologists received detailed tumor classifications within minutes, allowing them to make informed decisions while the patient remained in the operating room.
Experts describe this approach as revolutionary. It not only speeds up diagnosis but also reduces costs and eliminates the need for multiple separate tests. The trial reported a 100% success rate in providing accurate and rapid tumor classification. The cost per patient was about £450, with potential for further reduction as the technology scales. The results, published in Neuro-Oncology, highlight the method’s ability to deliver a fully integrated diagnosis within 24 hours. Both consultant neuropathologists and neurosurgeons involved in the study called it a game-changer for patient care.
Aspect | Details |
|---|---|
Trial Location | University of Nottingham and Nottingham University Hospitals NHS Trust |
Method | Nanopore-based sequencing with intraoperative methylome classification |
Diagnostic Time | Under 2 hours during surgery (vs. traditional 6-8 weeks) |
Number of Surgeries Tested | 50 brain tumor surgeries |
Success Rate | 100% accurate and rapid tumor classification |
Cost | Approximately £450 per patient, potentially less with scale-up |
Benefits | Faster, cheaper, eliminates need for multiple separate tests, delivers timely results |
Publication | Results published in Neuro-Oncology |
Clinical Impact | Considered revolutionary; aims for NHS Trust rollout |
Additional Notes | Provides detailed tumor classification within minutes; fully integrated diagnosis within 24 hours |
Expert Opinions | Described as a game-changer by Consultant Neuropathologist and Neurosurgeon |
Comparison to Standard Methods
Ultra-rapid droplet digital PCR (UR-ddPCR) stands out for its speed and reliability. Researchers tested UR-ddPCR on 22 brain tumor cases, analyzing 78 tissue samples. The results showed high agreement with standard ddPCR, a method known for its accuracy. UR-ddPCR detected key mutations, such as IDH1 R132H and BRAF V600E, down to 0.1% mutant DNA. This level of sensitivity matches the best available laboratory techniques.
UR-ddPCR provides results in about 15 minutes per assay, much faster than standard genetic tests.
The rapid turnaround does not compromise accuracy. UR-ddPCR matches standard ddPCR in detecting tumor cell percentages.
Surgeons can use this real-time data to guide their decisions during surgery, improving patient outcomes.
False positives and false negatives remain important considerations in any genetic test. A meta-analysis of over 750,000 non-invasive prenatal testing (NIPT) cases offers insight into these rates for ultra-rapid genetic testing. False positive rates range from 0.041% to 1.211%, depending on the condition tested. False negative rates stay below 0.01%. For example, trisomy 13 shows a false positive rate of about 0.048% and a false negative rate near 0.020%. The ratio of false positives to false negatives ranges from 7:1 to 30:1, meaning false positives are more common. These findings highlight the need for confirmatory testing after a positive or suspicious result.
Test Metric | False Positive Rate | False Negative Rate | Example (Trisomy 13) |
|---|---|---|---|
NIPT (meta-analysis) | 0.041%–1.211% | <0.01% | 0.048% / 0.020% |
Ultra-rapid genetic testing in brain tumor surgery demonstrates high accuracy and reliability. The technology matches standard methods in sensitivity and specificity, while delivering results in a fraction of the time. This combination of speed and precision supports its growing role in modern neurosurgery.
Surgical Decisions
Real-Time Mutation Guidance
Surgeons now use real-time genetic testing to guide their actions during brain tumor surgery. This technology provides immediate information about key mutations, such as IDH1 and BRAF, within minutes. Surgeons can adjust their approach based on the tumor’s genetic profile, balancing the need to remove as much tumor as possible with the goal of preserving healthy brain function.
Real-time mutation data helps surgeons decide how much tissue to remove. For example, patients with IDH-mutant gliomas benefit from more extensive tumor removal, which improves survival. Even small amounts of leftover tumor can affect outcomes for these patients. In contrast, for other tumor types, surgeons may focus on protecting critical brain areas.
The table below highlights how different rapid molecular techniques support intraoperative decisions:
Aspect | Summary |
|---|---|
Technique | Raman and mass spectrometry detect mutations like IDH in under 15 minutes. |
Clinical Impact | Surgeons tailor resection extent, improving patient outcomes and preserving function. |
Prognostic Value | IDH-mutant tumors respond better to aggressive removal. |
Implementation | These methods work directly in the operating room, requiring no extra sample processing. |
Surgeons also use real-time molecular data to identify tumor margins more accurately. This guidance allows them to maximize tumor removal while minimizing harm to healthy tissue. As a result, patients experience better recovery and improved long-term survival.
Tumor Cell Density
Ultra-rapid genetic testing remains effective across a wide range of tumor cell densities. The test can detect mutant DNA even when only a few tumor cells are present at the surgical margin. This sensitivity ensures that surgeons do not miss small clusters of cancer cells.
Aspect | Details |
|---|---|
Mutation Detection Sensitivity | |
Tumor Cell Density Range | From >1,300 cells/mm² (core) to <5 cells/mm² (margin) |
Turnaround Time | 14–17 minutes per assay |
Concordance | High agreement with standard ddPCR for cell percentage measurement |
Effectiveness | Maintains accuracy even at low tumor cell densities |
This capability helps surgeons achieve more complete tumor removal. Patients benefit from fewer residual cancer cells, reduced risk of recurrence, and faster recovery. Ultra-rapid testing supports precise, personalized surgery for every patient.
Complementing Other Tools
Integration with Imaging
Surgeons rely on many tools during brain tumor surgery. Intraoperative imaging, such as MRI and ultrasound, helps them see the tumor and plan the operation. Other advanced methods, like fluorescent-guided surgery, MALDI-MSI, and REIMS, give quick information about the tumor’s location and type. These tools can show where the tumor is and how it might differ from healthy tissue. However, they do not always reveal the tumor’s genetic subtype.
Ultra-rapid genetic testing in brain tumor surgery fills this gap. It gives surgeons real-time genetic information about the tumor. When combined with imaging, this testing allows doctors to match what they see on scans with the tumor’s genetic profile. This approach helps them plan the surgery more precisely and make better decisions during the operation. For example, studies show that using rapid genetic testing with imaging can lead to earlier diagnosis and better surgical planning. Surgeons can adjust their strategies quickly, which may reduce hospital stays and improve patient care.
Tip: Combining genetic testing with imaging gives a fuller picture of the tumor, helping surgeons balance tumor removal with protecting brain function.
Addressing Microscopic Infiltration
Many brain tumors spread tiny clusters of cells into nearby tissue. Imaging tools sometimes miss these small groups, making it hard to remove all the cancer. Pathology methods, like frozen section analysis, can help but often take too long or sample only small areas.
Ultra-rapid genetic testing detects even small amounts of tumor DNA at the edges of the surgical site. This sensitivity means surgeons can find and remove hidden tumor cells that imaging or standard pathology might miss. When used with tools like MALDI-MSI or Raman histology, genetic testing adds another layer of detail. Surgeons get both a map of the tumor’s spread and its genetic makeup. This combination supports more complete tumor removal and may help patients recover faster with fewer complications.
Tool/Method | What It Provides | Limitation | How Genetic Testing Helps |
|---|---|---|---|
Imaging (MRI, US) | Tumor location and size | Misses small clusters | Adds genetic detail |
MALDI-MSI, REIMS | Molecular and lipid profiles | Lacks genetic subtype info | |
Pathology | Cell type and structure | Slow, limited sampling | Rapid, sensitive mutation detection |
Together, these tools and ultra-rapid genetic testing in brain tumor surgery give surgeons the best chance to remove the tumor completely and safely.
Future Directions
Automation and Trials
Researchers continue to improve ultra-rapid genetic testing in brain tumor surgery. They focus on making the process faster, more accurate, and easier to use in the operating room. Current next-generation sequencing methods still face speed limitations. These methods cannot yet replace point-of-care tests because they take too long. Scientists work on new pipelines, such as ultra-rapid nanopore sequencing, which can deliver high-depth data in under two hours and diagnostic results in less than eight hours. This is up to 50% faster than previous methods.
Aspect | Current Challenge | Progress Being Made |
|---|---|---|
Speed of Testing | Standard NGS is slow | Faster sequencing pipelines under development |
Sample Preparation | Difficult with small samples | Improved protocols for better DNA quality |
Data Processing | Slow variant calling | Cloud and GPU computing for faster analysis |
Variant Prioritization | Hard to balance speed and accuracy | New methods for quicker, accurate results |
Automation stands as a key goal. Teams at leading hospitals, such as NYU Langone Health, plan to automate ultra-rapid droplet digital PCR and rapid sequencing. These efforts aim to make the technology more reliable and less dependent on human skill. Although no specific clinical trials on automation are registered yet, researchers expect new studies to begin soon. They also work to address challenges like sample availability and differences in testing methods.
Rapid genetic testing has already reduced diagnosis times from months to days. Early diagnosis helps doctors choose the best treatments and can improve patient outcomes.
Broader Cancer Applications
The benefits of ultra-rapid genetic testing in brain tumor surgery may soon reach other cancer types. Medical experts believe that as hospitals adopt these innovations, faster and more accurate diagnostics will help patients with many different cancers. The technology, now used for brain tumors, could become a model for near-patient, ultra-rapid sequencing in other fields.
Researchers see potential for pan-cancer tumor sequencing. They design new analytic tools and sequencing methods that can adapt to many cancer types. This approach could lead to earlier diagnosis, better treatment choices, and improved survival rates for patients with various cancers.
As technology advances, doctors hope to use ultra-rapid genetic testing to guide surgery and treatment for a wide range of cancers, not just those in the brain.
Ultra-rapid genetic testing in brain tumor surgery changes how doctors treat patients. Surgeons now receive fast and accurate results during operations. This speed helps them remove tumors more safely and completely. Patients benefit from better outcomes and shorter recovery times. Hospitals may soon use this technology for other cancers. Experts believe that real-time genetic data will shape the future of cancer care.
Fast and precise testing leads to safer surgeries and improved patient lives.
FAQ
What is ultra-rapid genetic testing?
Ultra-rapid genetic testing uses advanced technology to detect key mutations in brain tumor tissue within minutes. Surgeons receive results during surgery, which helps them make better decisions for each patient.
How accurate is ultra-rapid ddPCR compared to standard tests?
Ultra-rapid ddPCR matches the accuracy of standard ddPCR. Studies show high sensitivity and specificity, even at low tumor cell densities. Surgeons can trust the results for real-time guidance.
Can this technology replace traditional imaging or pathology?
Ultra-rapid genetic testing does not replace imaging or pathology. It complements these tools by providing genetic information that imaging cannot show. Surgeons use all available data for the best outcome.
Which mutations can ultra-rapid testing detect?
The test identifies important mutations, such as IDH1 R132H and BRAF V600E. These mutations help doctors classify tumors and choose the best surgical approach.
Is ultra-rapid genetic testing available for other cancers?
Researchers continue to expand this technology. Hospitals currently use it mainly for brain tumors. Future developments may allow its use in other cancer types as well.
See Also
A Comprehensive Overview Of Glioblastoma And Its Features
Exploring Craniopharyngioma And Its Important Defining Traits
Insights Into Large Cell Lung Cancer With Rhabdoid Features
Detailed Guide To Hemangioblastoma And Its Main Characteristics
Examining Malignant Fibrous Histiocytoma And Osteosarcoma Of Bone

